






In the ever-evolving landscape of neuroscience and medical research, certain milestones stand out as beacons of innovation and progress. At the USC Mark and Mary Stevens Neuroimaging and Informatics Institute (Stevens INI), we strive to be one such beacon, illuminating the path to a deeper understanding of the human brain. As we celebrate our journey, we embark on a reflective exploration of the past ten years—a decade marked by groundbreaking discoveries, collaborative endeavors, and the relentless pursuit of knowledge.
This book serves as a tribute to our commitment to advancing neuroimaging and informatics—two crucial pillars that have reshaped the contours of modern neuroscience—and to all those who have supported us along the way. The life-changing work we do is only possible because of the extraordinary support of our generous partners. At the core of our existence lies a clear and resolute mission—to unravel the intricacies of the human brain. Our commitment is not merely to academic pursuits but to the profound belief that advancing scientific knowledge is integral to the betterment of society.
Over the past ten years, we have translated this mission into tangible achievements by adopting cutting-edge technologies and cultivating an environment that encourages excellence. Our neuroimaging methodologies, complemented by innovative informatics approaches, have positioned us as pioneers in neuroscience. Collaboration has been a driving force behind our success. Throughout the pages of this book, you will encounter stories of interdisciplinary partnership, where minds from diverse backgrounds converge to create an intellectual landscape that fosters innovation. This collaborative spirit has propelled us toward our scientific goals and enriched our research environment.
As we celebrate our achievements, we turn our gaze toward the future with optimism and determination. The upcoming years promise continued growth and excellence as we remain committed to advancing research, embracing emerging technologies, and fostering relationships that transcend traditional boundaries. We hope this book will serve as an enduring tribute to the dedication of our researchers and staff, the great potential of our students and trainees, and the transformative generosity of our supporters.

USC establishes the INI with a founding team of 120 faculty, staff, and students from LONI.
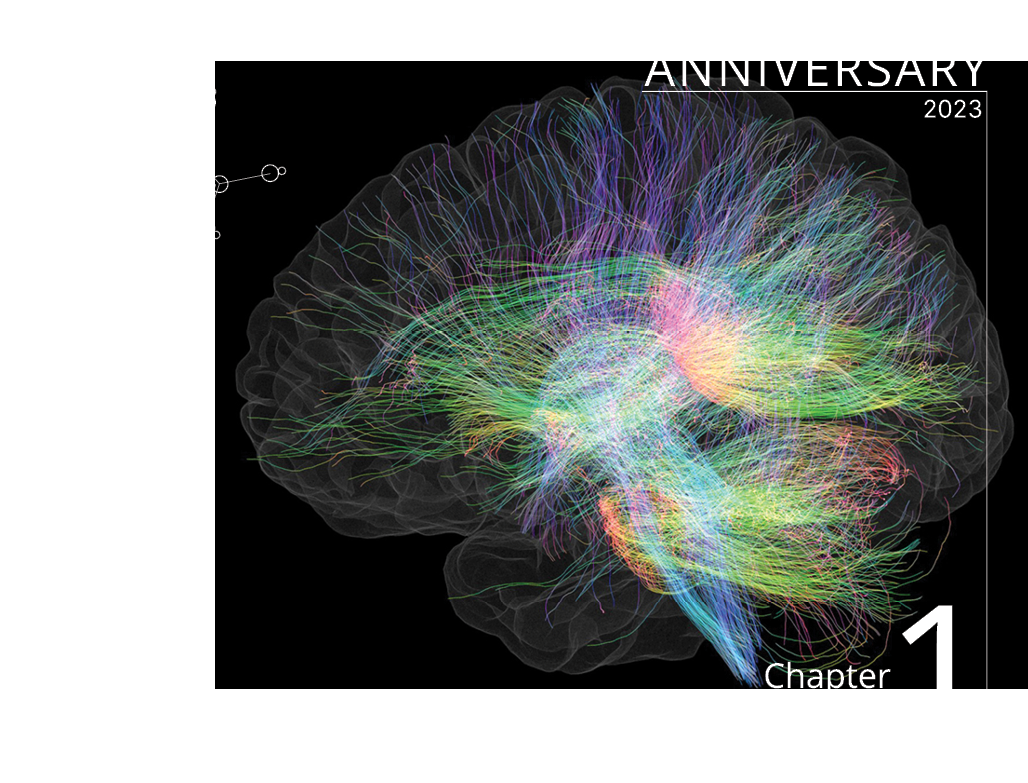
Chapter 1
Tractography
8
20
30
40
Chapter 2
Mouse Models
Chapter 3
Neurodegenerative Disorders
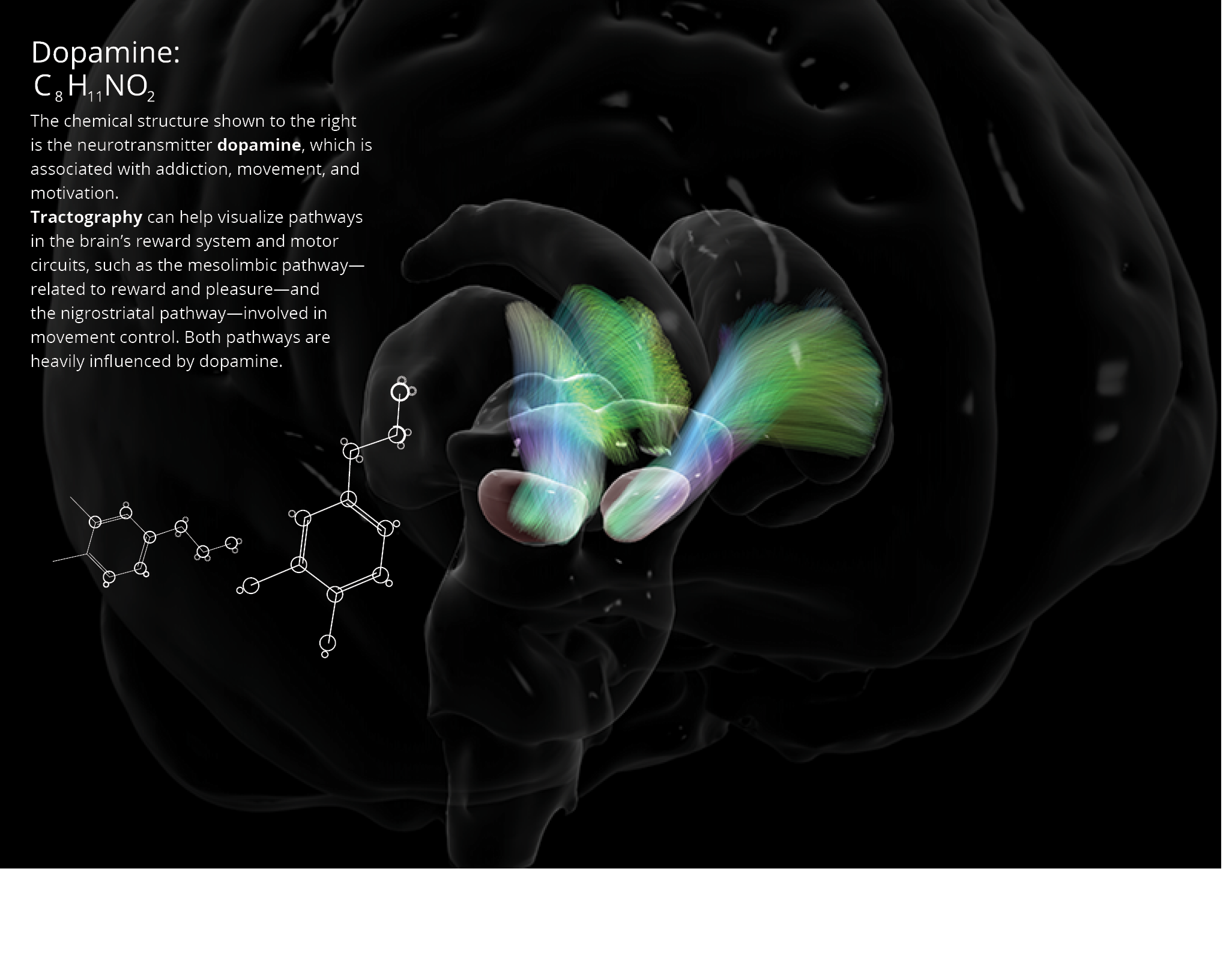
DOPAMINE
Chapter 4
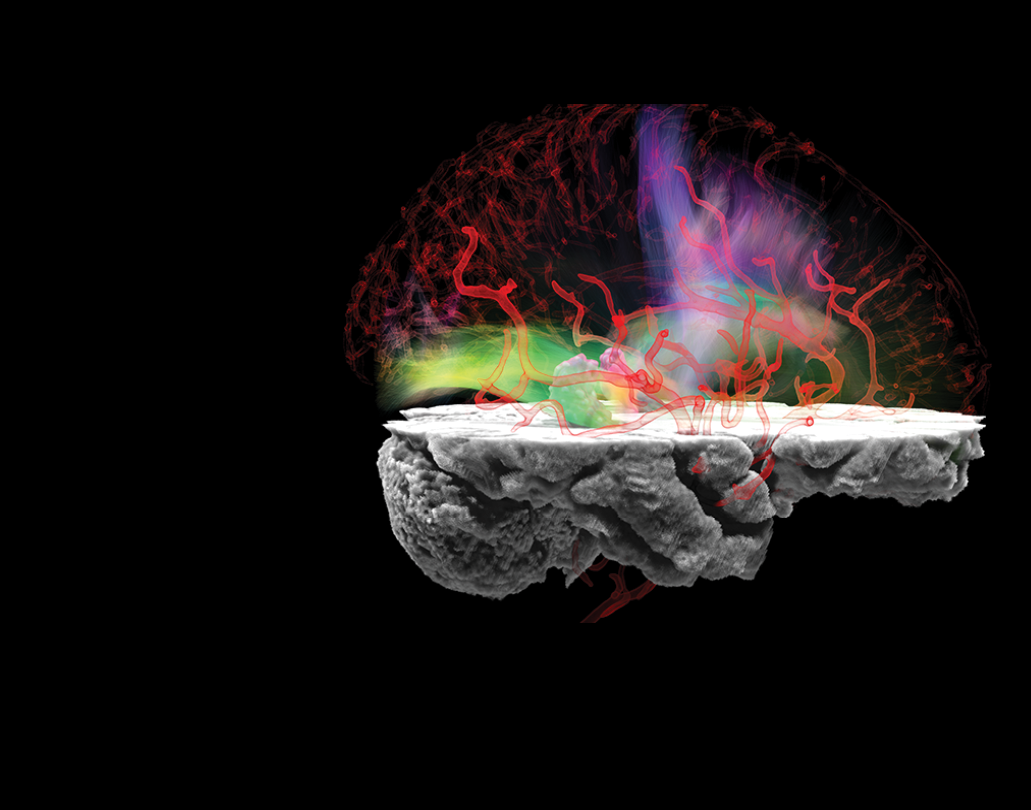
Vascular Imaging
Mark and Mary Stevens make a transformational gift to the INI that supports the custom design and buildout of Stevens Hall for Neuroimaging.
One of the world’s most powerful MRI scanners, a Siemens Terra 7T, is installed. The 7T is the first ultra-high-field scanner of its kind installed in North America.
2
0
1
3
2
0
1
5
2
0
1
7

SEROTONIN
HISTAMINE
Chapter 5
Big Data & Informatics
Chapter 6
Traumatic
Brain Injury, Epilepsy, Stroke
Chapter 7
Ultra-High-Field Imaging
Chapter 8
Education & Training
Chapter 9 Visualization
Chapter 10
The Future of Neuroimaging
50
62
74
84
94
106

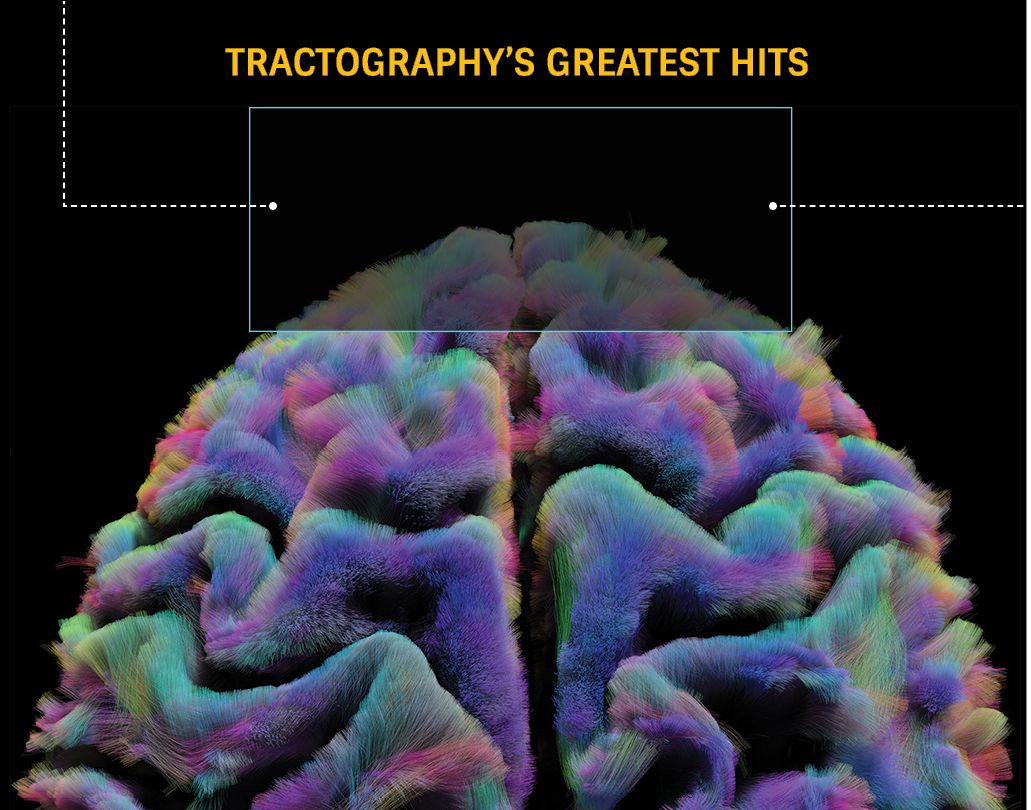
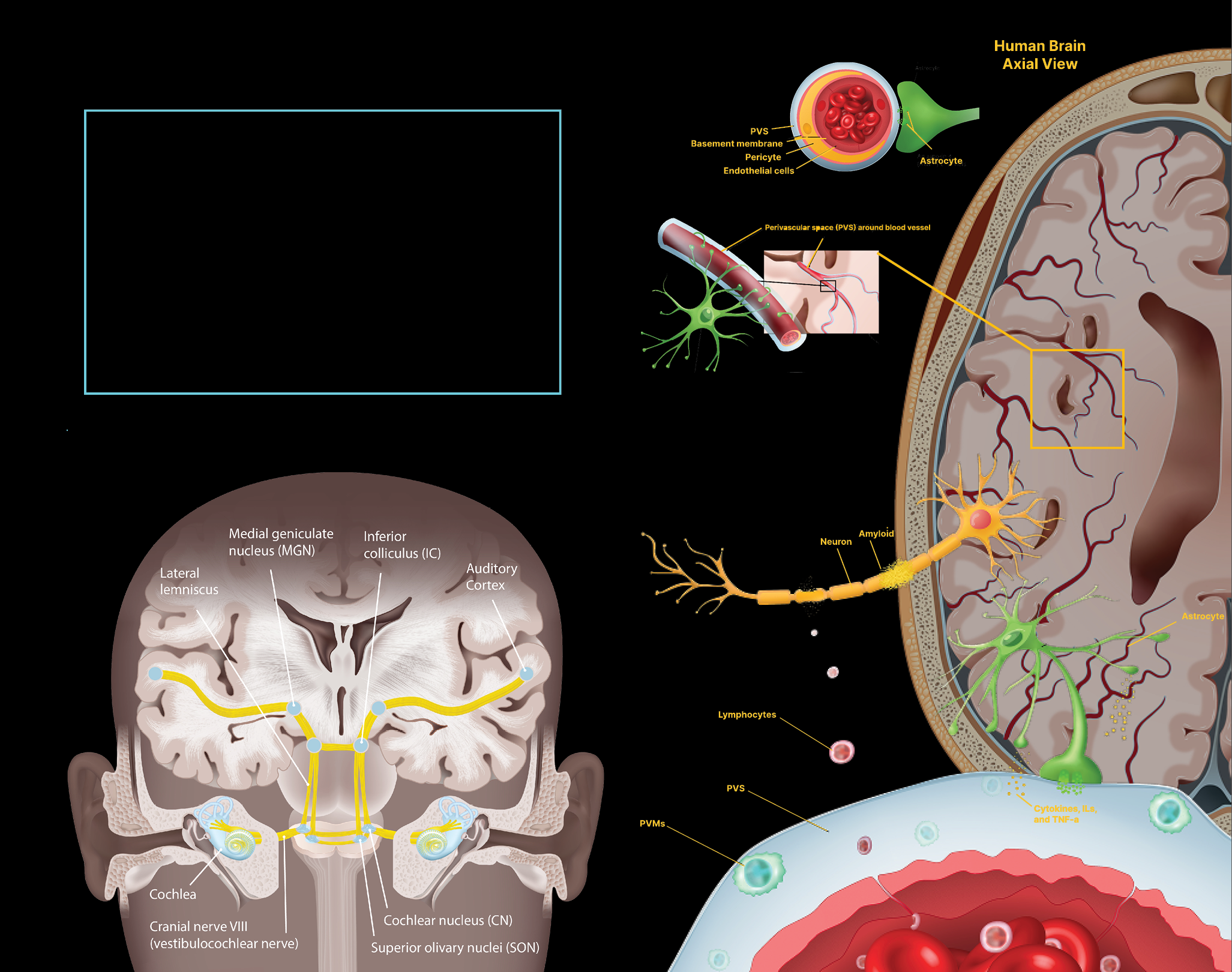
Illuminating brain connectivity
Mapping the brain in health and disease
Innovative methods and models



We specialize in tractography, a revolutionary technique that utilizes advanced imaging technologies to map and visualize neural pathways in the human brain.
By tracking the movement of water molecules along white matter tracts, tractography provides a 3D representation of the complex networks that facilitate communication between different brain regions. This method has significantly enhanced our understanding of brain connectivity.
10


To unravel the secrets of brain structure,
we pioneered an innovative approach using the cutting-edge technique known as multi-shell diffusion imaging. This revolutionary imaging method empowers researchers to delve deep into the intricacies of nerve fiber orientation within the brain. By enhancing our capacity to understand the complex network of connections in the brain, our research propels the creation of more precise models that help solve the mysteries of brain function.
11


By harnessing the power of parallel transport,
a mathematical concept derived from differential geometry, we elevated the precision and reliability of tractography. We crafted the parallel transport tractography technique to intricately map neural pathways within the human brain. This innovation holds the promise to significantly advance our comprehension of brain connectivity, laying the foundation for developing more precise models crucial for studying and treating a spectrum of neurological conditions.
12


Delving into the intricate landscape of neural connections,
we pioneered a method focusing on the outer layers of the brain, known as the superficial white matter. This technique provides a nuanced and precise understanding of the complex networks of nerve fibers near the brain’s surface. The implications are profound, offering a potential leap forward in our knowledge of brain structure and function and, in turn, opening avenues for enhanced diagnostic and therapeutic strategies.
13

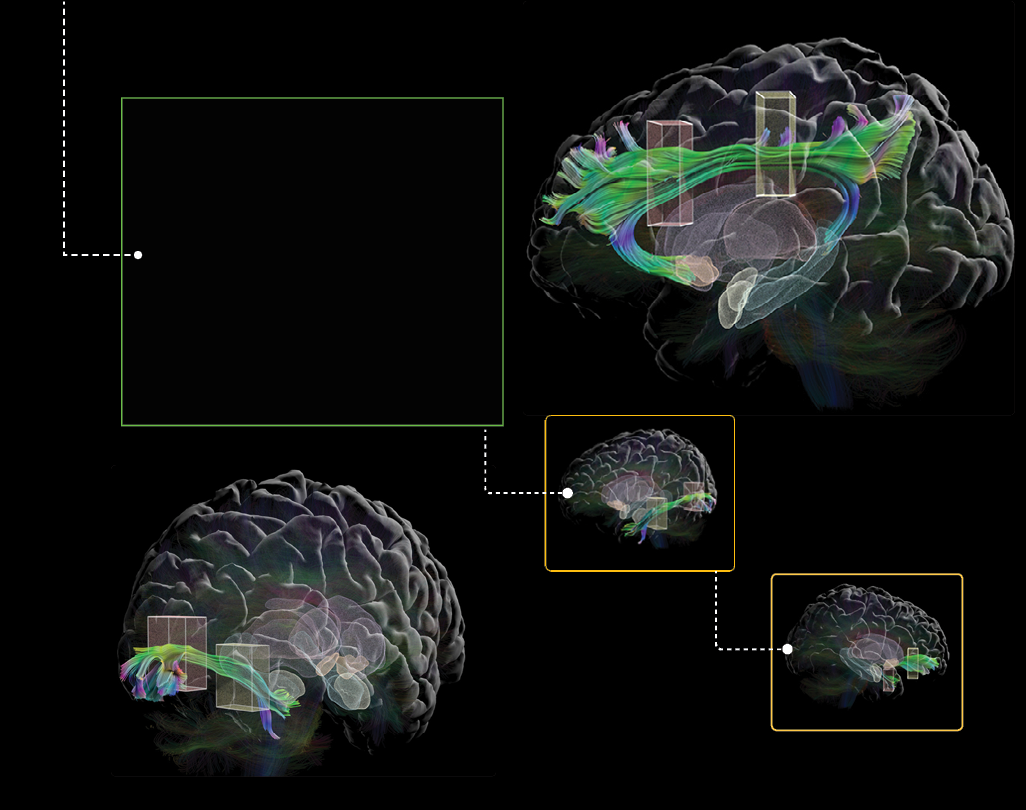
We reconstructed the visual pathway from the eyes to the brain
using cutting-edge imaging techniques. We created an automated method that promises efficiency and ensures more accuracy in tracing the intricate connections involved in visual processing. Beyond contributing to our understanding of the neural pathways governing vision, this work holds potential applications in elevating diagnostics and treatments for vision-related disorders.
14


In our quest to elucidate brain connections,
we devised a method employing iterative message-passing and pruning techniques. This meticulous approach enhances the accuracy of tracking and filtering brain connections, focusing not on individual threads but on the dynamic interplay within groups. This advancement is invaluable for gaining insights into how diverse brain regions communicate and connect, deepening our understanding of their collective functions.
15



Responsible for many vital functions of life,
the brainstem sends messages to the rest of your body to regulate balance, breathing, heart rate, and more. We constructed a detailed map that captures the variability and likelihood of different neural pathways in the brainstem. This atlas provides valuable insights into the structural connections within this crucial region of the brain. The work enhances our understanding of the complex network of pathways that regulate various bodily functions and contribute to overall brain function.
16


Mapping brain connectivity for Alzheimer’s disease holds the potential for advancing diagnostics and developing targeted treatments for the condition. In our exploration of brain connections, we’ve forged a detailed map shedding light on the interplay between the locus coeruleus and the transentorhinal cortex. This atlas takes on particular significance in the context of Alzheimer’s, offering insights into how communication between these brain regions may influence the disease’s progression.
17








18


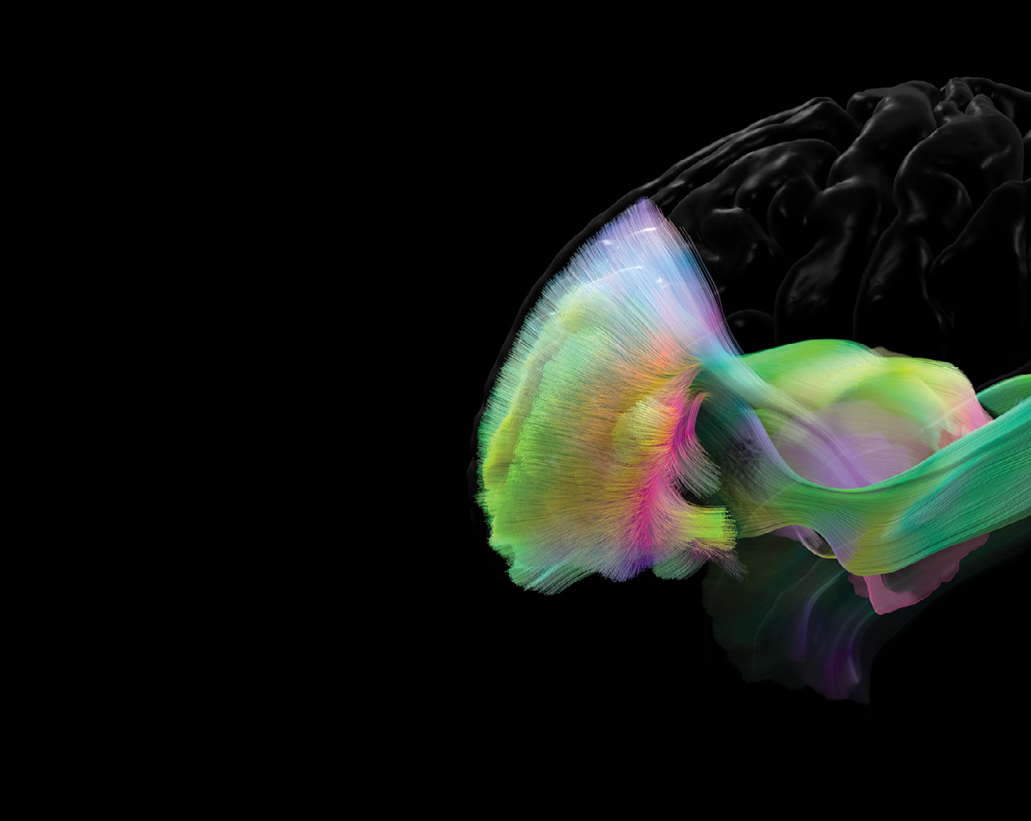
In the next decade, more sophisticated and reliable tractography techniques will be developed by computational researchers. Several more advanced tracking algorithms are under active development in our research group. These techniques will be based on solid mathematical principles and a deeper understanding of the relationship between tractography and brain anatomy. Rigorous and thorough validation of tractography will continue to be pursued in the field, even though this is a highly challenging task. We envision robust and consistent tractography techniques will be successfully applied to generate highly effective biomarkers for various brain disorders such as Alzheimer’s disease. The integration of tractography-based and other imaging markers by artificial intelligence models represents more exciting opportunities for our future work.
19

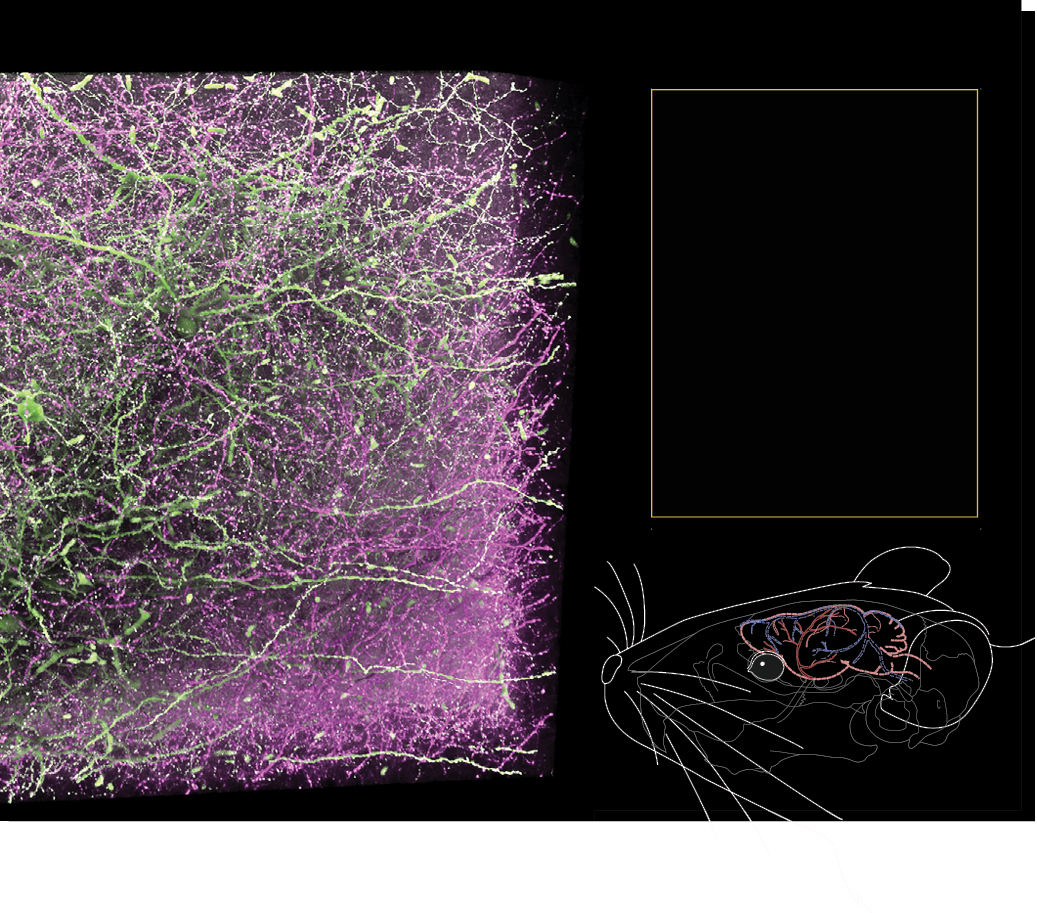
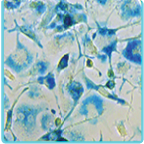
Fluorescent staining is a common technique used in neuroscience to allow scientists to visualize specific cellular structures and molecules. In this technique, various chemicals or dyes emit fluorescence when excited by particular wavelengths of light, allowing for the visualization of different types of cells, proteins, or other biological molecules.
Classifying cell types
Mapping visual pathways
Supporting clinical investigations



We are home to a cross-disciplinary group of experts working to develop a multimodal, multiscale connectome and cell-type map of the mammalian brain using advanced tracing, imaging, and computational methods. The mouse connectome is essential for unraveling the mysteries of brain function, advancing our understanding of neurological disorders, inspiring developments in artificial intelligence, and providing a foundation for developing innovative therapeutic approaches. We develop neuroanatomic and neuroinformatic approaches to understand connectivity patterns in both health and disease through our mouse connectome work.
22


OF MICE AND MEN
The Mouse Connectome Project is our NIH-funded venture that aims to create a complete mesoscale connectivity atlas of the mouse brain and subsequently generate its global neural networks. The project provides researchers with a better understanding of how various brain structures communicate with one another. Ultimately, the hope is that understanding healthy brain connections will offer clues to better treating the brain’s abnormal connections and disconnections, which are involved in numerous neurological conditions, including Alzheimer’s disease, Parkinson’s disease, and autism spectrum disorder.
23
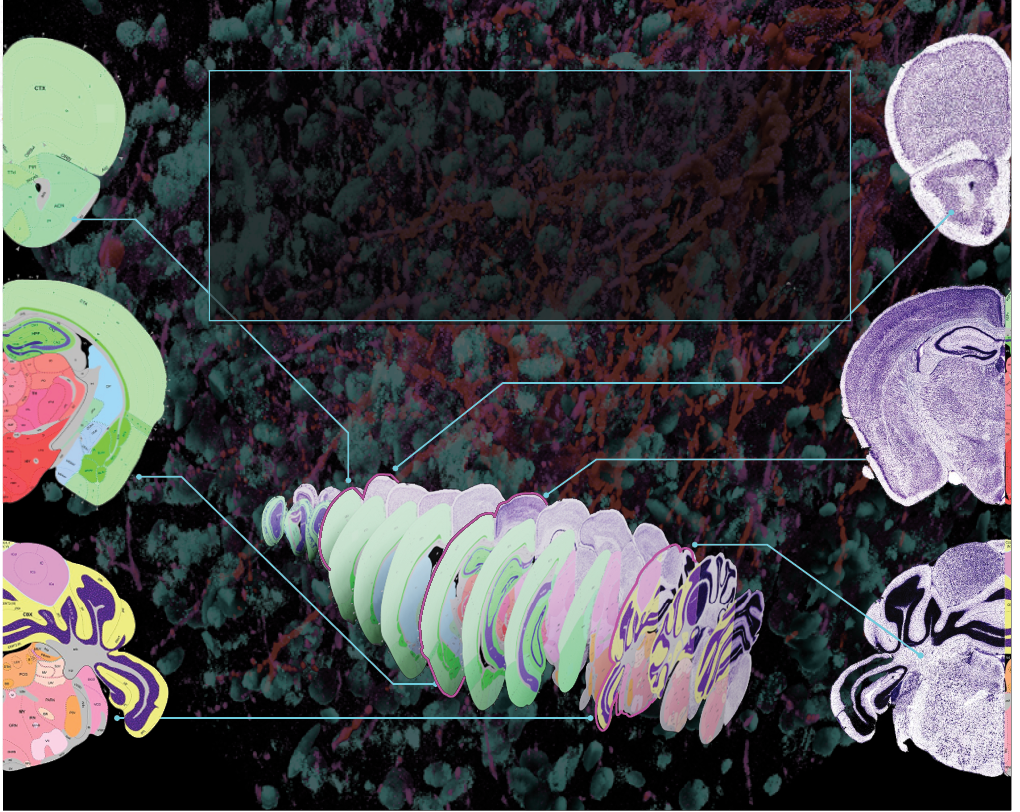

ALL OVER THE MAP
Using gene expression patterns and viral tracers, we published the most comprehensive map of the hippocampus to date using fluorescent tracers and 3D animation to show structures, nerve connections, and functions in vivid detail. Our analysis provided key insights into how the hippocampus is organized as a whole structure and how different parts of the hippocampus can mediate functions like spatial navigation, memory, emotion, and hormonal regulation. The mouse Hippocampus Gene Expression Atlas is a crucial resource scientists can use to better understand the human hippocampus and its degeneration in Alzheimer’s disease.
24

CELL CENSUS BUREAU
We are partners with the BRAIN Initiative as a part of the BRAIN Initiative Cell Census Network (BICCN), which anatomically characterizes cell types across the brain. Through the BICCN, we have released two groundbreaking resources: a comprehensive classification of cell types in the mammalian primary motor cortex and a detailed atlas of the region. The primary motor cortex is the part of the brain responsible for skilled movement, from typing to tap dancing. Our work establishes a critical resource to drive clinical investigations into movement disorders such as Parkinson’s and Huntington’s disease.
25
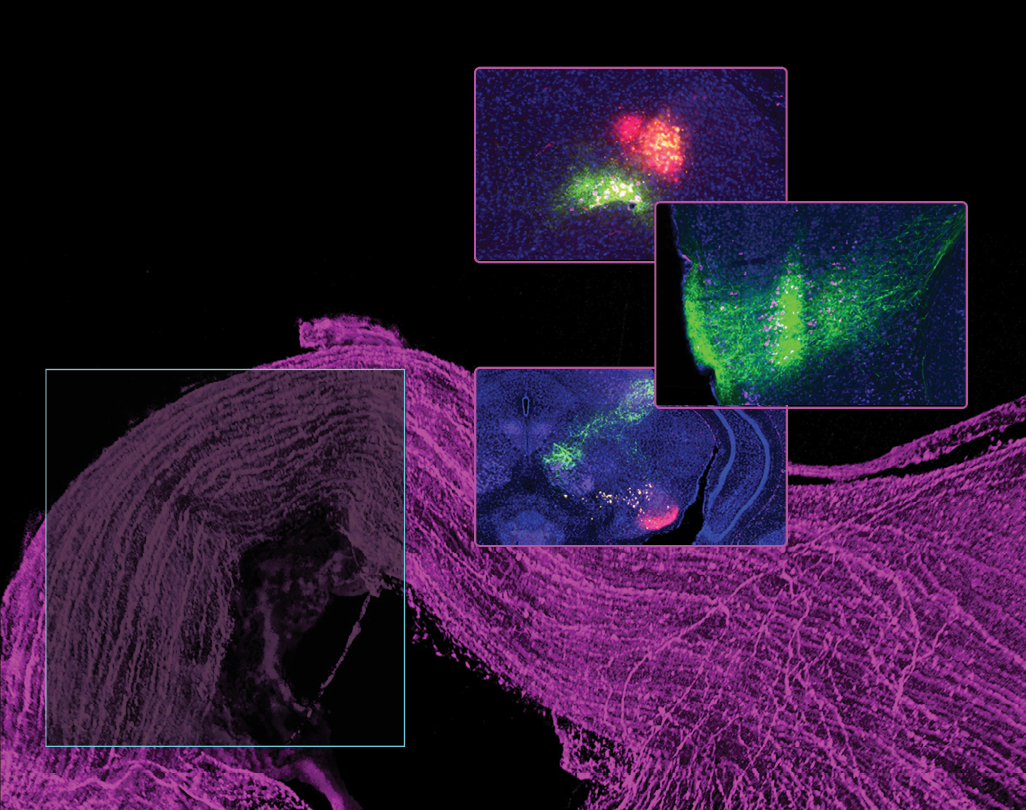

SEEING THE BIGGER PICTURE
We examined connectivity between several key parts of the mammalian visual system. We found new pathways in the mouse brain that relay visual signals from the eye to the cortex, where conscious perception occurs. These pathways may underlie a phenomenon known as ‘blindsight’ in patients who retain some visual abilities despite losing vision due to brain damage. Understanding blindsight can advance our knowledge of brain plasticity, guide rehabilitation strategies for visual impairments, map visual pathways, and inform the development of artificial intelligence systems related to vision.
26


COMMIT TO MEMORY
The subiculum is an integral part of the brain linked to memory, and it is particularly affected by Alzheimer’s disease. In our research with mice, we discovered a hidden layered structure in the subiculum. However, because the orientation of the hippocampus varies between species, it was unclear whether this structure was the same in humans. By analyzing data from the Allen Human Brain Atlas, we found that the human subiculum also has these layered patterns, similar to mice. We also found that certain molecular boundaries in the human subiculum match the differences we can see in high-resolution brain scans. Additionally, we noticed similarities and differences in the gene activity of specific cells within the subiculum layers.
27




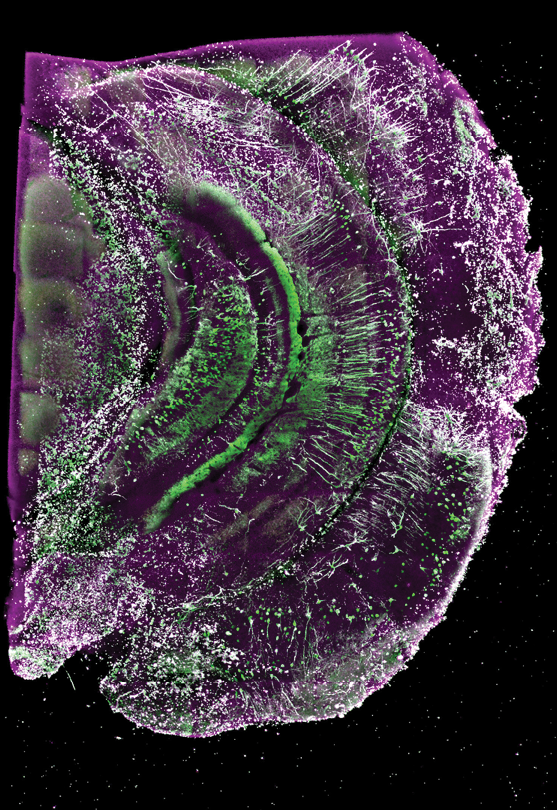









DIP IN THE GENE POOL
We have incorporated the MERSCOPE imaging system into our work. This microscopy imaging system uses an approach called MERFISH (Multiplexed Error Robust Fluorescence In Situ Hybridization) to fluorescenvtly label hundreds of genes within a single brain tissue section, which allows us to identify brain cell types and understand how their expressions are changed by neurodegenerative diseases like Alzheimer’s disease.
We will use this method on mouse and human brain tissue. MERFISH’s capabilities in single-cell resolution, multiplexing, quantitative analysis, spatial context, and detection of rare events make it a promising technology for advancing research into neurodegenerative diseases. It allows researchers to explore the molecular and cellular aspects of these diseases in greater detail, potentially leading to new insights, diagnostic markers, and therapeutic targets.
28



With continuous advancements in genetic engineering techniques, researchers can create increasingly sophisticated mouse models that closely mimic human neurological conditions. The emerging field of connectomics, focusing on mapping the intricate networks of neuronal connections, is leveraging mouse models to decipher the mouse connectome. There may be a growing emphasis on integrating connectomic data with other ‘omics’ datasets, such as genomics and proteomics, providing a more holistic understanding of how genetics and molecular processes influence neural connectivity. This integration could deepen comprehension of the molecular mechanisms underlying neural circuit development, plasticity, and disease. The near future holds great promise for using mouse models to unlock fundamental insights into brain function, neurological disorders, and potential therapeutic interventions.
29


State-of-the-art artificial intelligence methods
Worldwide, multi-year studies
Early biomarker discovery




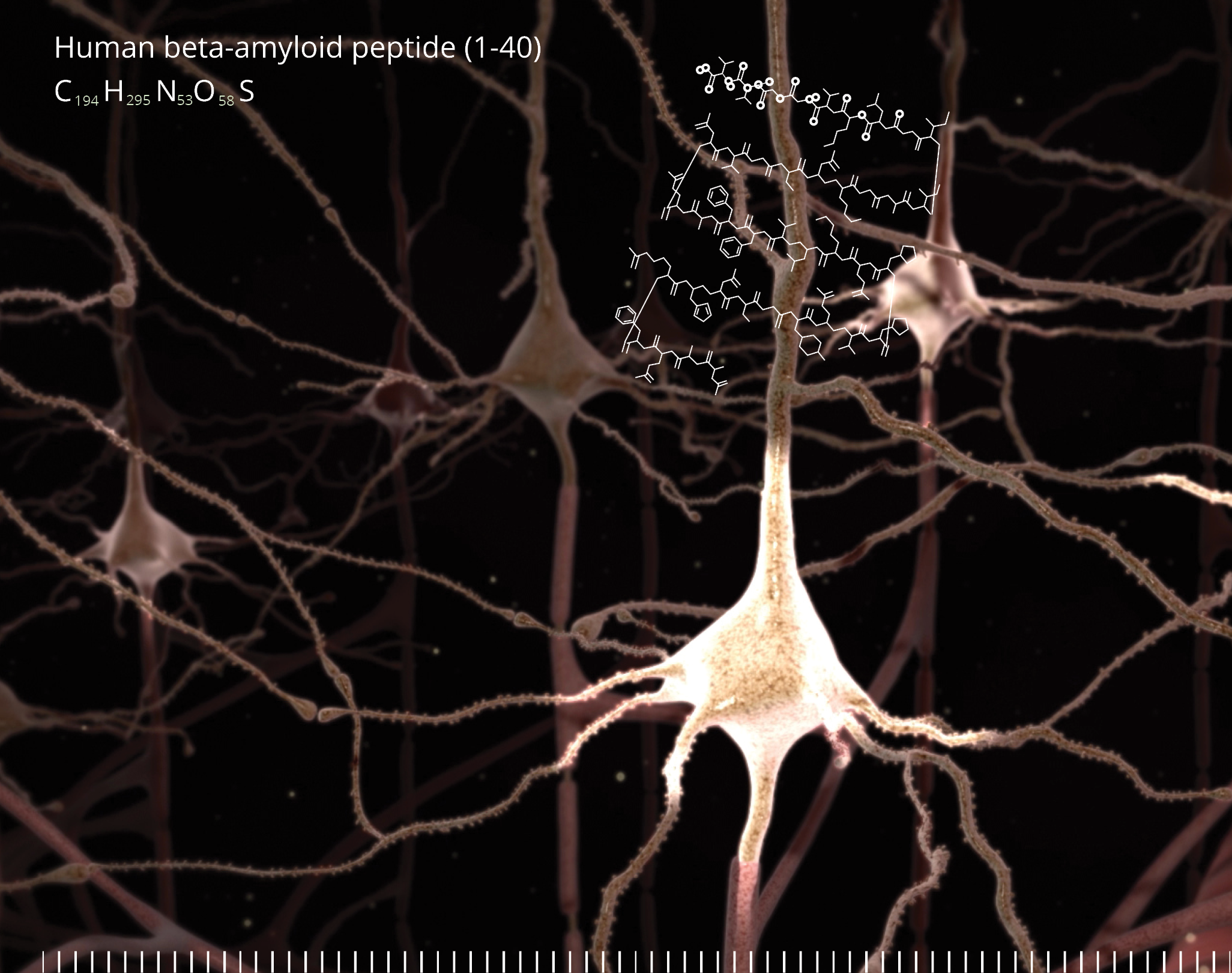
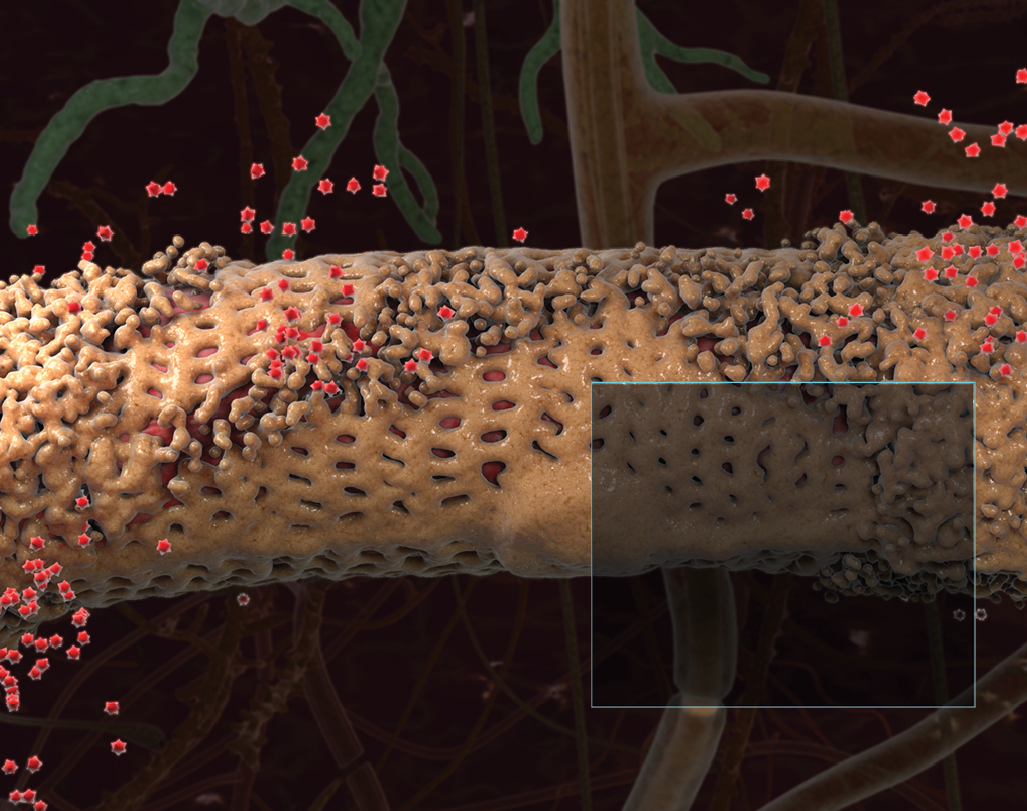
Amyloid is protein aggregates characterized by a specific structure, typically arranged in fibrous formations known as amyloid fibrils. These fibrils are insoluble and display a cross-beta sheet structure, a distinct molecular arrangement. The formation of amyloid fibrils is associated with several neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease.
In neurodegenerative disorders, the accumulation of amyloid fibrils in the brain is a hallmark feature. For instance, in Alzheimer’s disease, amyloid-beta (Aβ) peptides accumulate to form plaques, which are one of the pathological markers of the disease. These plaques are thought to disrupt cell-to-cell communication and activate immune system cells that trigger inflammation and devour disabled cells.
The relationship between amyloid and neurodegenerative disorders is a key area of focus in drug development. Many therapeutic strategies aim to prevent the formation of amyloid fibrils, promote their clearance, or mitigate their effects on neural cells. For example, drugs may be designed to inhibit enzymes involved in amyloid-beta production in Alzheimer’s disease or to enhance the clearance of amyloid from the brain.








25
Much of our research is focused on revealing blood and imaging biomarkers, as well as indicators of genetic risk for neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. We achieve excellence by combining results and findings and then continuing to aggregate data across projects of increasing magnitude. Using high-field imaging tools and our deep learning strategies, we are leveraging our collaborative networks to tackle these debilitating diseases from every possible angle. We believe this approach will yield more accurate results and accelerate the pace of discovery.
32

Artificial intelligence is revolutionizing healthcare.
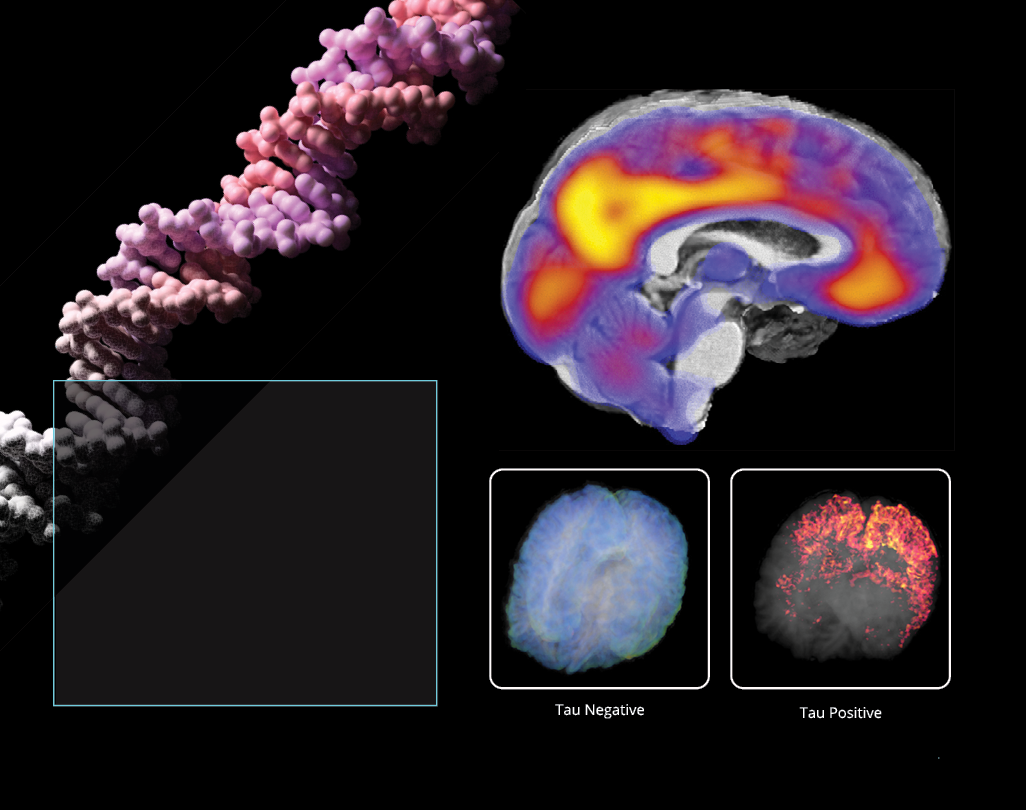
We use artificial intelligence to identify genomic motifs and features associated with Alzheimer’s disease. This includes using AI to merge and calibrate several types of imaging—MRI, amyloid-PET, tau-PET, and vascular imaging—to identify Alzheimer’s disease subtypes and relate these subtypes to specific genomic predictors and outcomes. We are also working to create a drug prioritization system to identify drugs to repurpose based on the genomic markers discovered in our work.
33

Alzheimer’s disease is currently ranked as the seventh leading cause of death in the US
A massive problem like Alzheimer’s—which affects nearly 50 million people worldwide—requires bold solutions. Our project, “Ultra-scale Machine Learning to Empower Discovery in Alzheimer’s Disease Biobanks,” known as AI4AD, develops state-of-the-art artificial intelligence methods and applies them to giant databases of genetic, imaging, and cognitive data collected from AD patients. Our team of experts creates algorithms that analyze data at a previously impossible scale and enable the discovery of new features in the genome that influence the biological processes involved in AD.
34






More than 10 million people worldwide are living with Parkinson’s disease.
We lead a global study that unites researchers and data from more than 20 countries to answer some of the most pressing questions about Parkinson’s disease. ENIGMA-PD studies whether treatments, including drugs such as L-Dopa and surgical therapies such as deep brain stimulation, can slow down this progression of brain tissue loss and clinical decline. We investigate genetic factors that contribute to PD risk, with a particular focus on how different genes increase the risk of developing PD in individuals with European versus Asian ancestry.
35

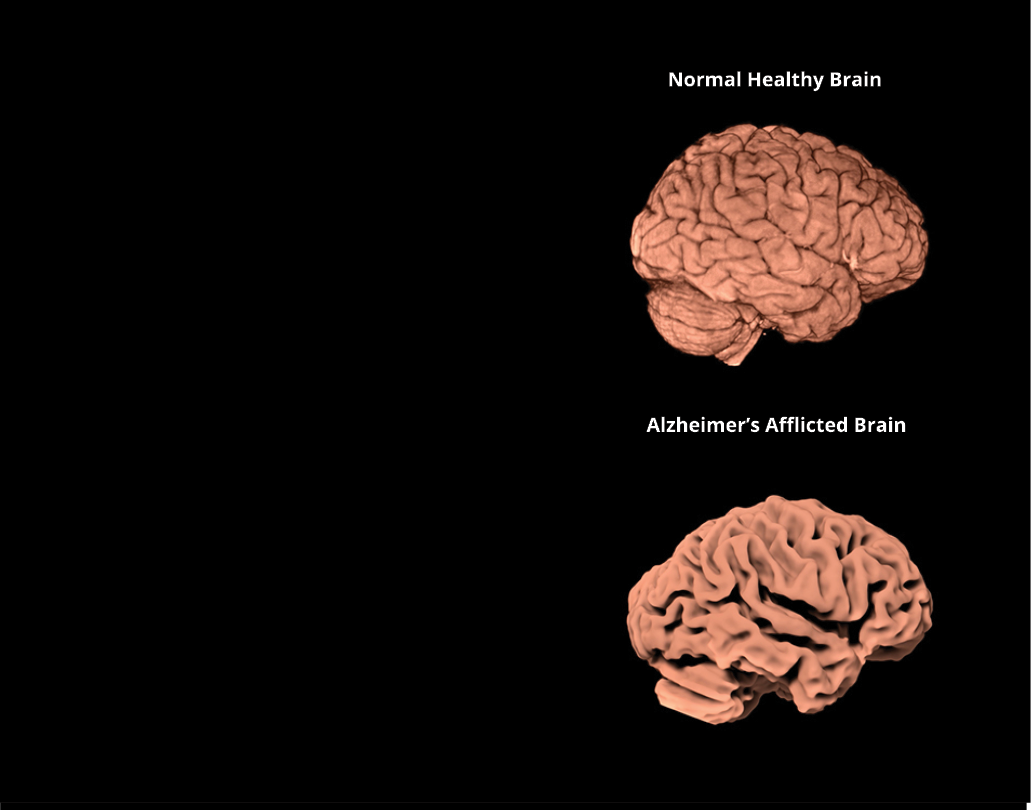
We revealed that white women genetically predisposed to Alzheimer’s are more susceptible than men between ages 65 and 75.
In addition to genetics and environmental factors, Alzheimer’s is highly associated with metabolic syndrome.
Our discovery is important because it counters long-held beliefs about who is at greatest risk for the disease and when and highlights how clinical trials could be weighted toward women—a susceptible part of the population—to help scientists more rapidly identify effective drug interventions to slow or cure Alzheimer’s. In the future, doctors who want to prevent Alzheimer’s may intervene at different ages for men and women.
Some of our studies examine the link between physical activity, metabolic risk, genetic risk, and brain aging. We have received results indicating that metabolic risk and inflammation both play a crucial role in the brain atrophy characteristic of Alzheimer’s disease and other dementias. Biological indicators of disease—like the elevated cholesterol levels and hypertension that typically precede heart attacks—are key for prevention and early intervention. However, no such biomarker exists for Alzheimer’s disease, meaning diagnosis happens only after significant brain damage has occurred. One of our top priorities is to advance the global search for the earliest biomarkers in neurodegenerative diseases, an essential step toward developing effective therapies and prevention strategies.
36


What causes Parkinson’s remains largely unknown, and no two people have the same exact symptoms.
The Michael J. Fox Foundation’s flagship study of Parkinson’s disease biomarkers, the Parkinson’s Progression Markers Initiative (PPMI), strives to change this grim fact. Following more than 1,000 participants in 33 clinical sites worldwide, we host all data from the longitudinal study and have contributed to several recent breakthroughs, including developing machine learning models to aid in the early diagnosis of PD. We continue to help profile biological and clinical changes across the spectrum of disease with an emphasis on signals in at-risk individuals to identify the disease and intervention points as early as possible
37




African American and Hispanic individuals are at a higher risk for developing Alzheimer’s and other dementias.
Research has revealed that Hispanic and African American populations experience a significantly greater risk of developing mild cognitive impairment and Alzheimer’s disease than non-Hispanic whites. We’re addressing health disparities in Alzheimer’s disease research through the Health and Aging Brain Study – Health Disparities (HABS-HD), the most comprehensive study of Alzheimer’s disease among diverse communities ever conducted. HABS-HD is the first of its kind—a large-scale study using the AT(N) framework with a cohort that is more representative of the ethnic diversity we live in.
38


There have been significant strides in identifying specific molecules and protein signatures in the blood that correlate with the onset and progression of conditions like Alzheimer’s and Parkinson’s diseases. The quest for reliable blood biomarkers is gaining momentum due to their minimally invasive nature and potential for early disease detection. As precision medicine becomes more integral to healthcare, these blood biomarkers could serve as accessible tools for screening, diagnosing, and monitoring neurodegenerative disorders. Collaborative efforts across research institutions and the healthcare industry are crucial for validating and implementing these biomarkers into clinical practice, offering the prospect of earlier interventions and personalized treatment strategies. This marks a promising shift towards more effective diagnosis and management of neurodegenerative disorders.
39











































Hemoglobin is a protein found in red blood cells that carries oxygen from the lungs to the rest of the body and returns carbon dioxide from the body to the lungs to be exhaled. Its ability to bind to and release oxygen is what allows our cells and organs to function properly.
Nitric oxide is a crucial regulator of vascular health. Although not a neurotransmitter in the traditional sense, it acts as a signaling molecule in the endothelium—the lining of blood vessels—to promote vasodilation, which is the widening of blood vessels. This relaxation of blood vessel walls is important for maintaining normal blood pressure and ensuring adequate blood flow.
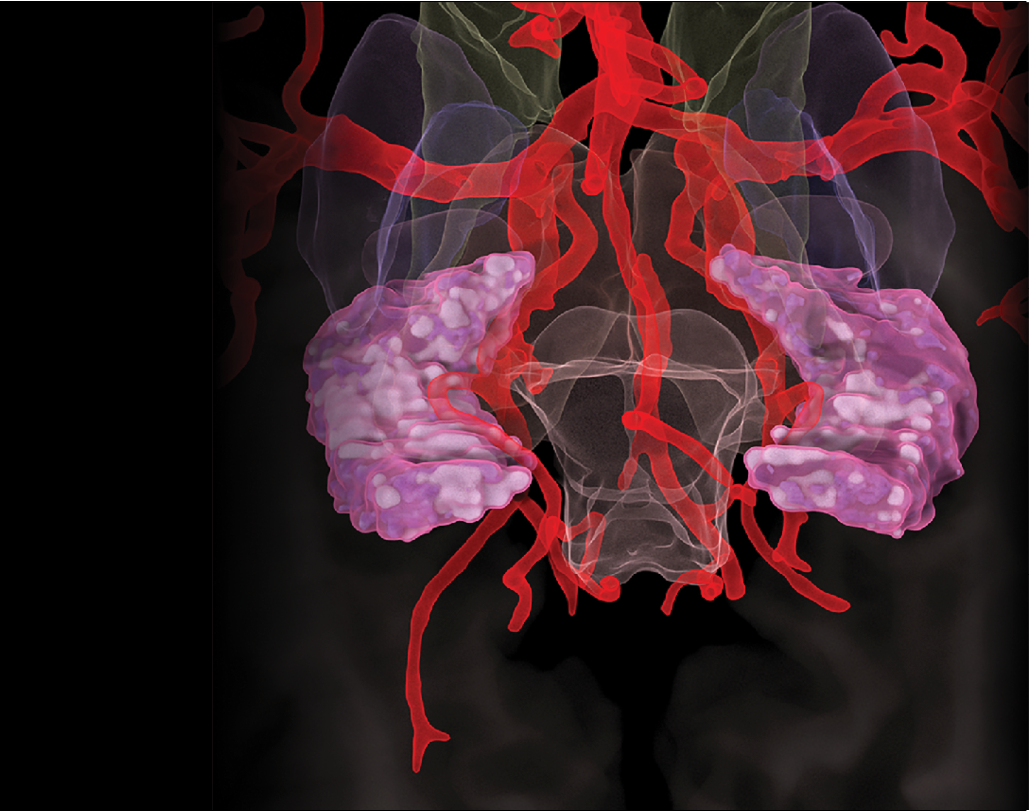
Targeting vascular brain health
Building nationwide collaborations
Discovering noninvasive imaging biomarkers





Studying the brain’s blood vessels gives researchers a window into how fluids and molecules move around. When something goes wrong, it can also help them understand the basis of various neurological diseases. We are leaders in an array of collaborate efforts to understand how the brain’s vasculature and perivasculature contribute to health and disease.
42


LET IT FLOW
We participate in a national consortium, the Vascular Contributions to Dementia and Alzheimer’s Disease (VCDAD) study, a multimodal assessment of the role of neurovasculature in cognitive decline. The study is a comprehensive research program on perivascular spaces—key for waste clearance in the brain—and numerous close-up investigations of how fluids travel through the brain, including via the blood-brain barrier. Our team is contributing our studies of the permeability of the blood-brain barrier, which increases with age and is believed to be closely linked to dementia.
43
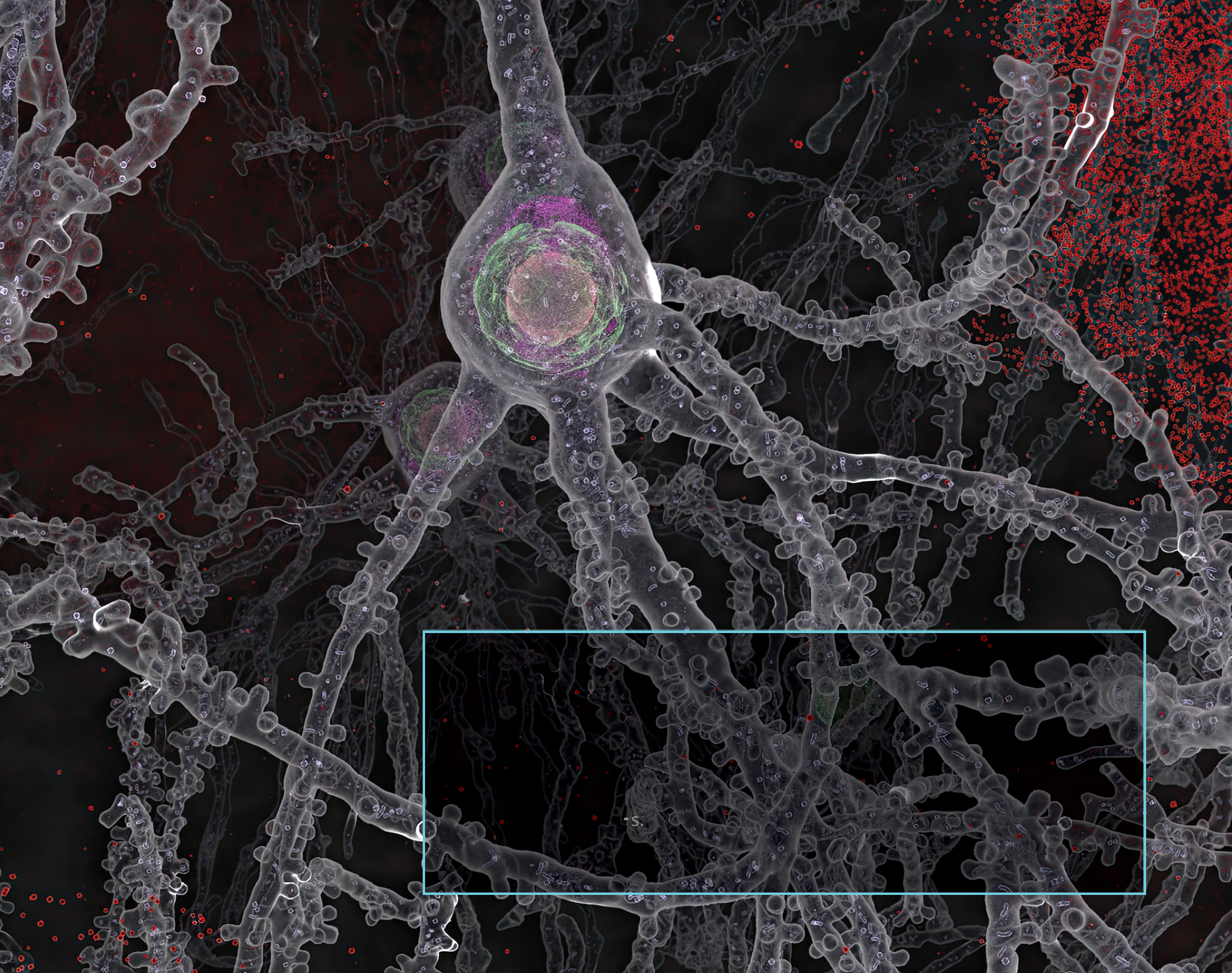

TANGLED UP IN TAU
One of our Alzheimer’s disease studies found that impaired blood flow in the brain is correlated with the buildup of tau tangles, a hallmark indicator of cognitive decline. The work suggests that treatments targeting vascular health in the brain — as well as amyloid plaques and tau tangles — may be more effective in preserving memory and cognitive function than single-target therapies.
44


MAPPING ACROSS THE LIFESPAN
We developed a new perivascular space (PVS) mapping technology that has been applied to more than 13,000 high-resolution magnetic resonance imaging brain scans to build maps of PVS at various stages across the lifespan. PVS are fluid-filled regions in the brain involved in clearing waste and toxins. Our findings show that PVS varies drastically from person to person — variations that are partly influenced by body mass index, gender, and even time of day.
45

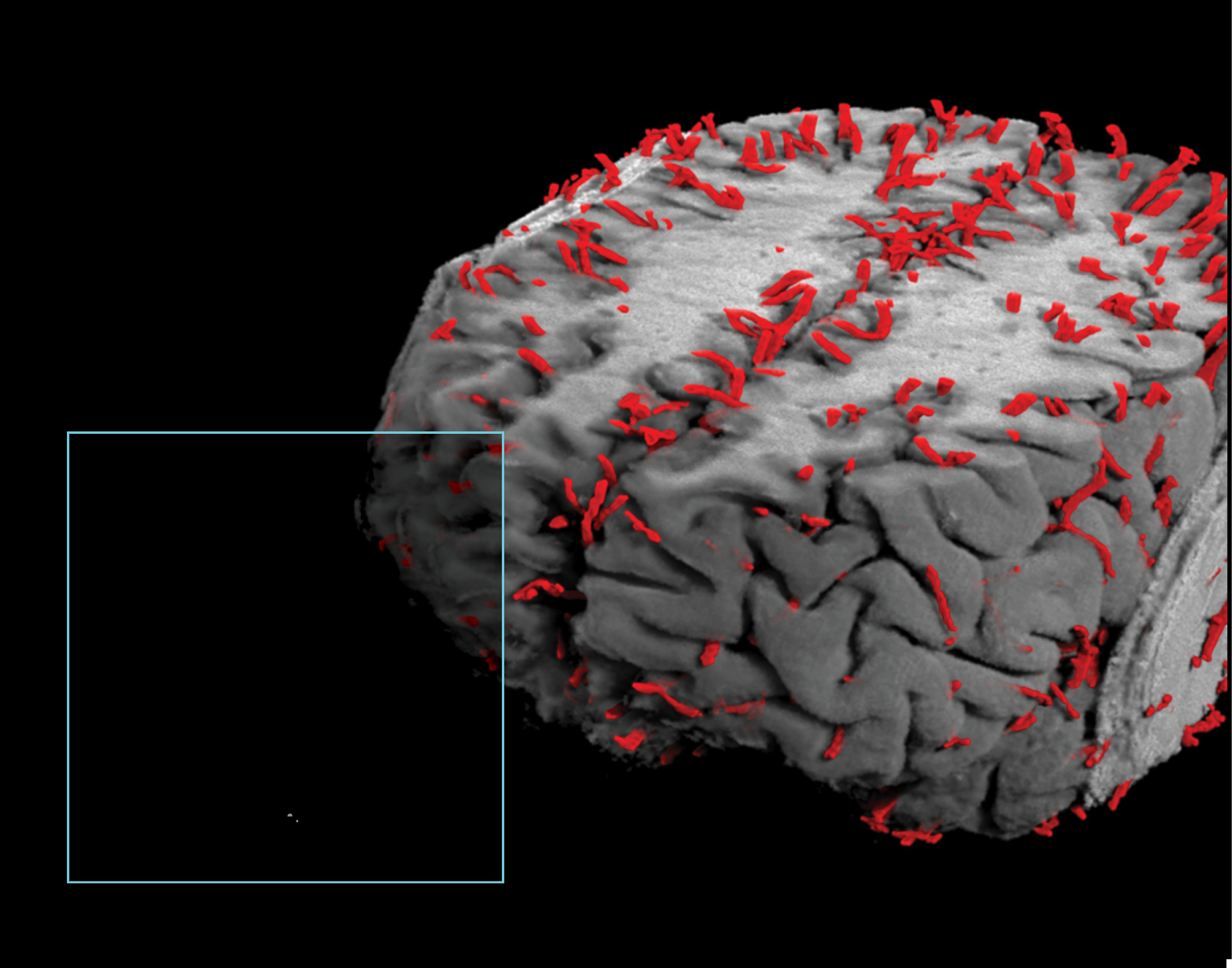
FINDING THE LEAK
Our application of high-resolution imaging of the living human brain shows for the first time that the brain’s protective blood barrier becomes leaky with age, starting at the hippocampus, a critical learning and memory center that is damaged by Alzheimer’s disease. The study indicates it may be possible to use brain scans to detect changes in blood vessels in the hippocampus before they cause irreversible damage, leading to dementia in neurological disorders characterized by progressive loss of memory, cognition, and learning.
46


SMALL VESSELS, BIG IMPACT
We participate in a nationwide consortium known as Biomarkers for Vascular Contributions to Cognitive Impairment and Dementia (MarkVCID) that aims to identify and validate biological markers that predict the onset of dementia by studying the brain’s smallest blood vessels. We use MRI to measure the stiffness of blood vessels, which is an indicator of hypertension and other vascular diseases. Our goal is to have a suite of biomarkers ready for clinical trials so the various candidate treatments for vascular cognitive impairment can be tested and hopefully shown to be effective.
47
CHANGE OF PACE
We developed a new MRI method called diffusion-prepared arterial spin labeling (DP-ASL) that can detect subtle changes in blood-brain barrier (BBB) dysfunction by measuring water exchange across the BBB. The BBB is a network of blood vessels and tissue that is made up of closely spaced cells and helps keep harmful substances from reaching the brain. We tested whether DP-ASL could provide a noninvasive imaging biomarker for early BBB problems associated with cerebral small vessel disease, with promising results.
48

Future technological innovations, such as high-resolution imaging techniques and noninvasive imaging modalities, will play a pivotal role in unraveling the complexities of the brain’s vascular system. Advanced imaging tools, including magnetic resonance imaging (MRI) and functional MRI (fMRI), will offer unprecedented insights into cerebral blood flow dynamics, aiding researchers in understanding the intricate interplay between neural activity and vascular responses. Real-time imaging and three-dimensional reconstructions will become more commonplace, enabling a more comprehensive exploration of cerebrovascular architecture and dynamics. These advancements in vascular imaging hold the potential to enhance our understanding of neurodegenerative diseases and cerebrovascular disorders, ultimately contributing to the development of more targeted and effective diagnostic and therapeutic interventions.
49



It started with LONI. The Laboratory of Neuro Imaging (LONI) has a rich history deeply rooted in brain mapping and atlasing, data collecting and sharing, and neuroimaging informatics. Founded in 1983 by Arthur W. Toga, LONI quickly established itself as a pioneering institution in the field of neuroimaging research due to its multidisciplinary approach, applying big data and informatics to advanced neuroimaging. Over the years, LONI has evolved, expanding its collaborations globally and embracing cutting-edge technologies to further unravel the mysteries of the brain, making profound impacts on neuroscience.
Sophisticated computational tools
Collaborative global projects
Premier data-sharing platforms



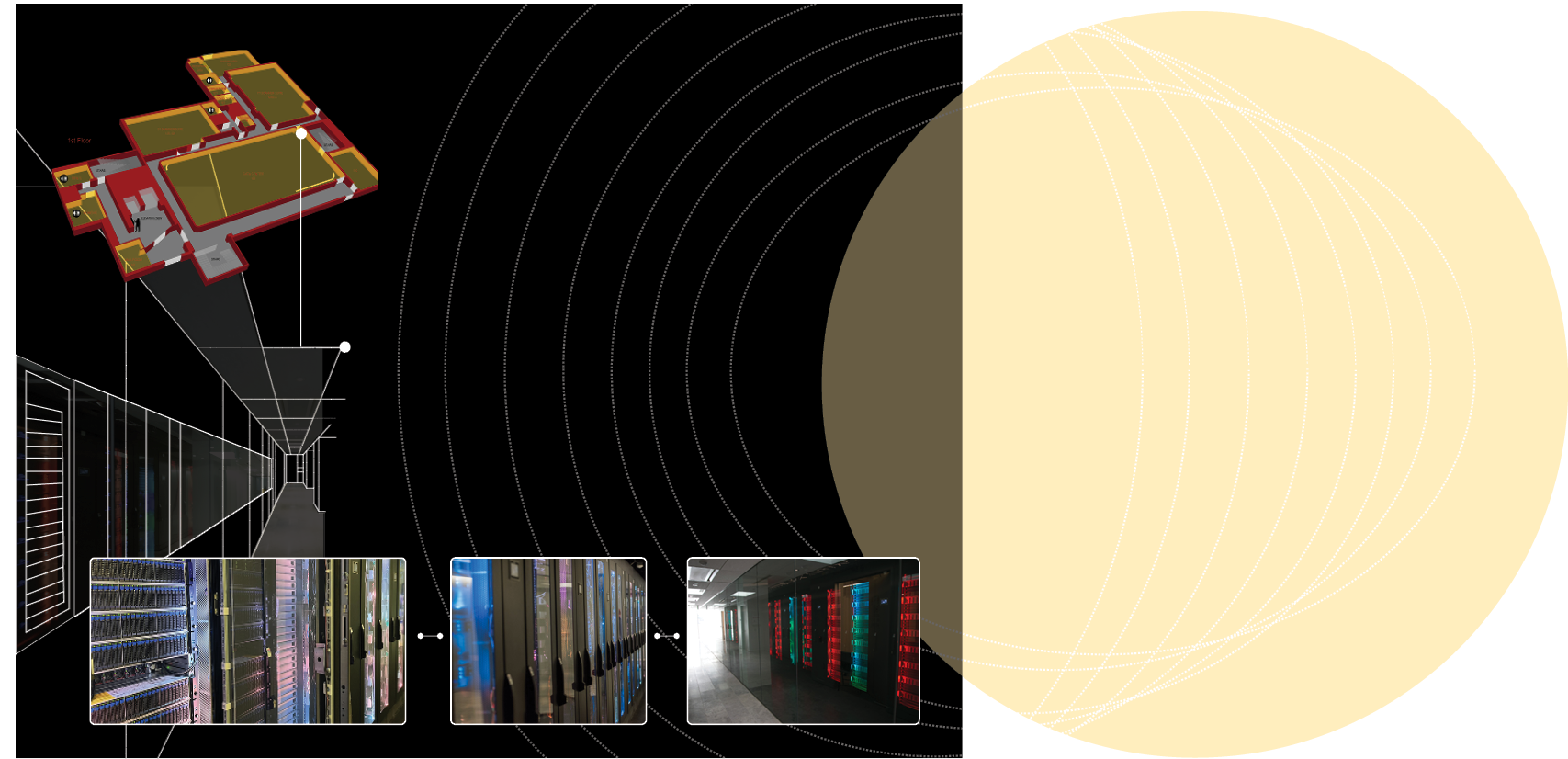
We house a 3,000-square-foot data center that supports hundreds of local, national, and global big data investigations in neuroscience. The facility boasts cutting-edge networking, storage, and computational capabilities to ensure data are secure yet easy for researchers to access and share. The data center has grown to reach 18 petabytes of data storage wvith 4,096 processor cores and 38 terabytes of aggregated memory space.
1 PB = 1,048,576 GB = 1,048 TB
The Library of Congress collection is estimated to be around 17 petabytes.
If 1 GB is the size of the earth,
then 1 PB is about 0.8 of the sun.
3,000
18
38
4,096
Sq. Ft. Data Center
Patabytes of Data Storage
Terabytes of aggregated memory space
Processor cores





52


Our immense repository of data is used to conduct diverse research and facilitate collaboration on projects across the globe. As informatics leaders, we apply sophisticated mathematical and computational tools paired with revolutionary visualization methods to gain new insights from our wealth of data. We lead dozens of collaborative global projects and maintain the world’s most extensive training data collection for machine learning, including imaging, genetics, biosample, cognitive, and electrophysiology data.
MORE DATA,
MORE DISCOVERY
53






SEEING IS BELIEVING
We created the world’s largest archive of neuroimaging data—the Image and Data Archive (IDA)—which provides tools and resources for de-identifying, integrating, searching, visualizing, and sharing a diverse range of neuroscience data and facilitates collaborations between scientists worldwide. The IDA has grown to more than 159,000 users, with over 369 million downloads and more than 1.9 million uploads.
Female
Male
Collected Data
Modality
Study
Design
Research
Focus
Longitudinal
Inteventional
Cross-Sectional
Other
Huntington’s
Parkinson’s
Stroke
Depression
PTSD
Austism
Control
Down’s
Aging
Cerebrovascular
TBI
Alzheimer’s
Fronto Temporal
MRI
CT
SPECT
PET
Exam &
Assessment
Genetic
Other
Biospecimen
Demographics
Sex
54




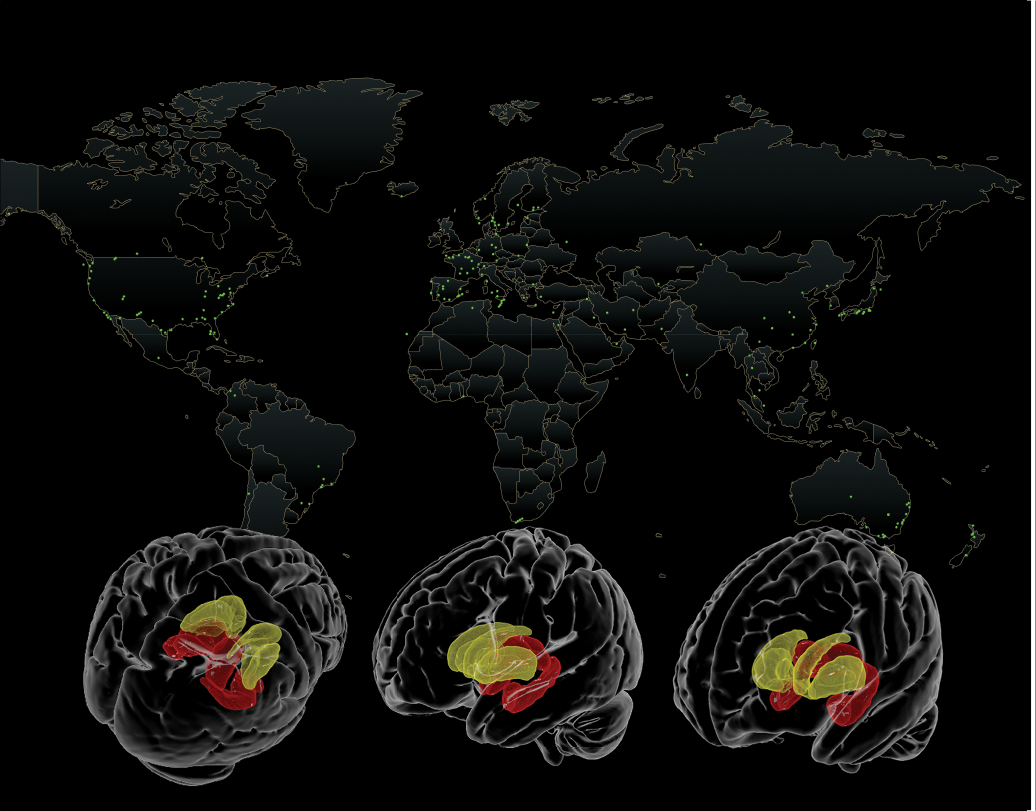
UNITED WE SOLVE
The Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium unites more than 2,000 scientists in over 40 countries to pool data and expertise to tackle pressing questions about more than 30 brain diseases. This consortium has created detailed maps of how schizophrenia, bipolar disorder, depression, post-traumatic stress disorder, autism, and other conditions affect the brain—leading to a better understanding of the underlying biology. ENIGMA brings together expert researchers from multiple disciplines to help crack the neurogenetic code.
55
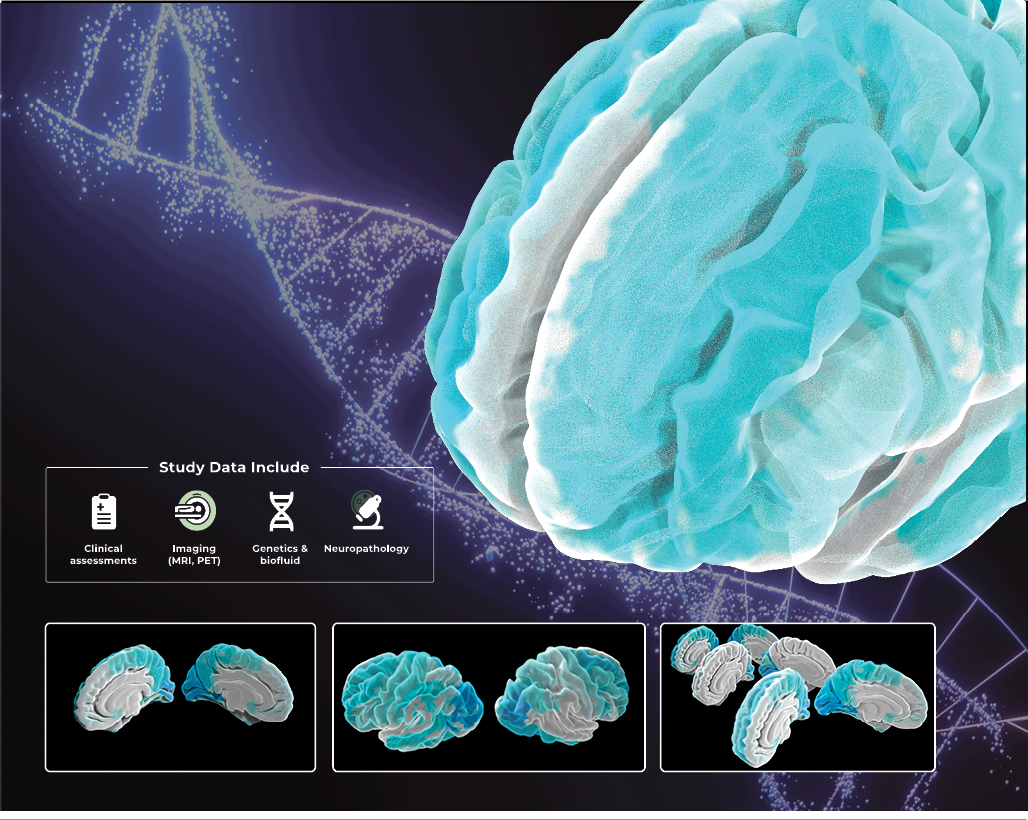
MISSION POSSIBLE
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) is a longitudinal multi-center study designed to develop clinical, imaging, genetic, and biochemical biomarkers for detecting and tracking Alzheimer’s disease. We host all the data for ADNI to support the overarching mission of detecting AD at the earliest possible stage, creating new diagnostic methods, and sharing the data we collect with researchers all over the globe. Since its launch in 2004, ADNI has made significant contributions to AD research by enabling the sharing of data between researchers around the world to facilitate discovery.
56


BIG DATA IN HARMONY
We designed and built the Data Archive for the BRAIN Initiative (DABI) — a shared repository that ingests, harmonizes, aggregates, stores, visualizes, and disseminates invasive human neurophysiology data collected by investigators from the BRAIN Initiative. The BRAIN Initiative is a prestigious public-private partnership led by the National Institutes of Health that aims to use new technologies to revolutionize the study of the human brain. The archive aids researchers in organizing, sharing, and analyzing their data.
57


SHARING THE WEALTH
We created LONI Resource (LONIR) to support neuroimaging researchers using comprehensive imaging analysis to investigate brain structure, function, and physiology in health and disease. LONIR provides the neuroscience research community with access to the algorithms and tools we develop, with the goal of fostering research relationships. With access to new techniques and information, investigators around the world can enhance their current imaging analysis programs.
58


SO MUCH TO GAAIN
We designed, built, and continue to manage the Global Alzheimer’s Association Interactive Network (GAAIN), the Alzheimer’s Association’s premier data-sharing platform, which uses a unique “federated” model to connect researchers around the globe by collecting and analyzing data on Alzheimer’s disease. Researchers can access vast datasets and conduct preliminary analyses, while data owners maintain rights and control over their information. This year, GAAIN is also celebrating its tenth anniversary—take a look at the progress:


Alzheimer’s Association GAAIN grant awarded
First version of GAAIN Interrogator (GAAIN1) online
Centiloid dataset for converting amyloid PET measurements to a standardized scale available online
Second version of GAAIN Interrogator (GAAIN3) online
Eight awards granted for the GAAIN Exploration to Evaluate Novel Alzheimer’s Queries (GEENA-Q) funding program
GAAIN Interrogator and ADDI workbench analysis pipelines and brain image processing feature initialized
GAAIN listed as an NIH-supported data sharing resource
More than 600,000 participants across more than 60 Data Partners represented in GAAIN
2013
2014
2017
2018
2022
2023
2024






59


60


Big data and informatics are poised to revolutionize scientific research and medicine in profound ways. Integrating massive datasets from various disciplines will facilitate interdisciplinary collaborations and accelerate discoveries. Advanced analytics will enable researchers to uncover hidden patterns and correlations, leading to breakthroughs in genomics, drug discovery, and much more. In medicine, big data will drive personalized healthcare by leveraging large-scale patient data to tailor treatments based on individual genetic, lifestyle, and environmental factors. The use of predictive analytics will enhance disease prevention and early intervention strategies. The healthcare industry will also see improved operational efficiency by integrating electronic health records and data-driven decision-making. The near future holds immense promise for leveraging big data and informatics to advance scientific understanding, enhance medical treatments, and transform healthcare delivery on a global scale.
61


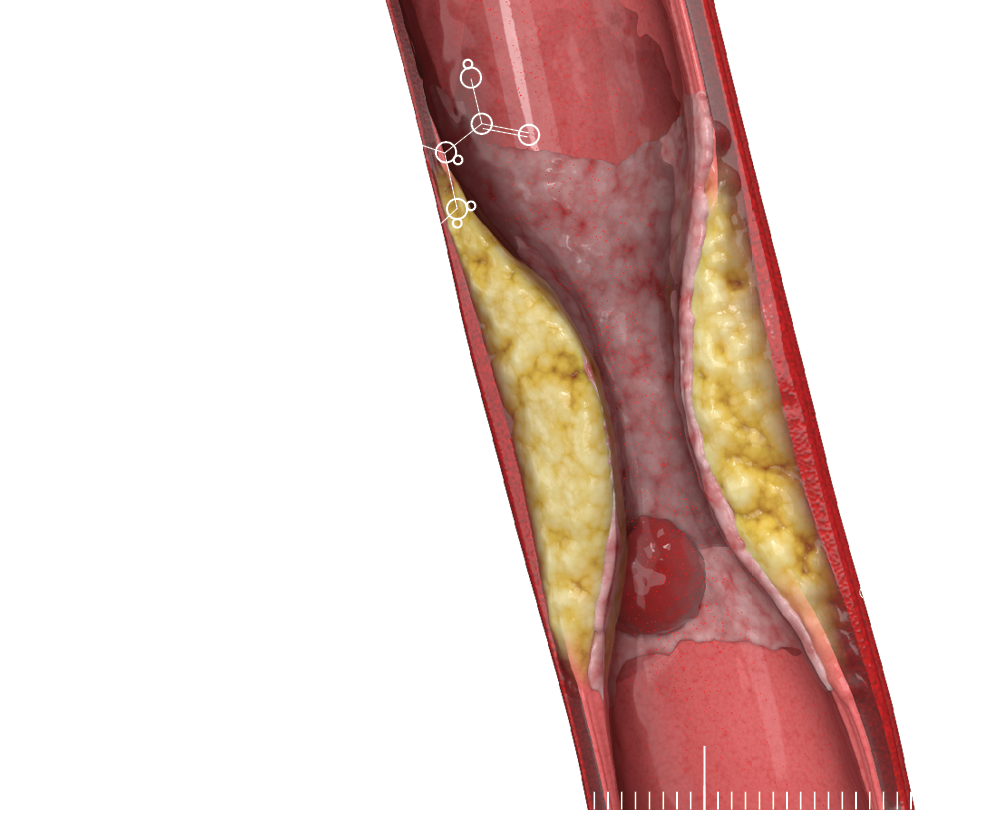
Glutamate is a key neurotransmitter in the brain, but too much of it can be released, causing excitotoxicity. This over-activation of glutamate receptors can damage and kill nerve cells. In TBI, this is due to physical damage. In epilepsy, this is due to abnormal electrical activity. In stroke, interrupted blood flow leads to glutamate buildup, damaging brain cells. Researchers are actively studying ways to mitigate glutamate excitotoxicity.
Advancing neurorehabilitation
Improving lesion detection
Understanding brain age

We are advancing research and discovery by using novel imaging technologies to study the structural and functional changes in the brain associated with conditions like traumatic brain injury (TBI), epilepsy, and stroke. Our specialists also utilize sophisticated informatics tools to analyze large datasets, facilitating a deeper understanding of the underlying mechanisms and aiding in the development of innovative diagnostic, treatment, and rehabilitation strategies for these neurological disorders.
64


Epilepsy and seizure disorders affect more than 5 million Americans.
One of our studies found that epilepsies, a group of disorders known to vary greatly in their causes and symptoms, share key structural foundations in the brain. The study shows that although epilepsy is a highly heterogeneous disorder with dozens of different causes, presentations, and treatments, multiple common forms of epilepsy do share similar neuroanatomical underpinnings. For example, we found that the thalamus, a central sensory relay region, and the precentral gyrus, which is the brain’s primary motor region, both show consistent abnormalities across all the various types of epilepsies in our studies.
65
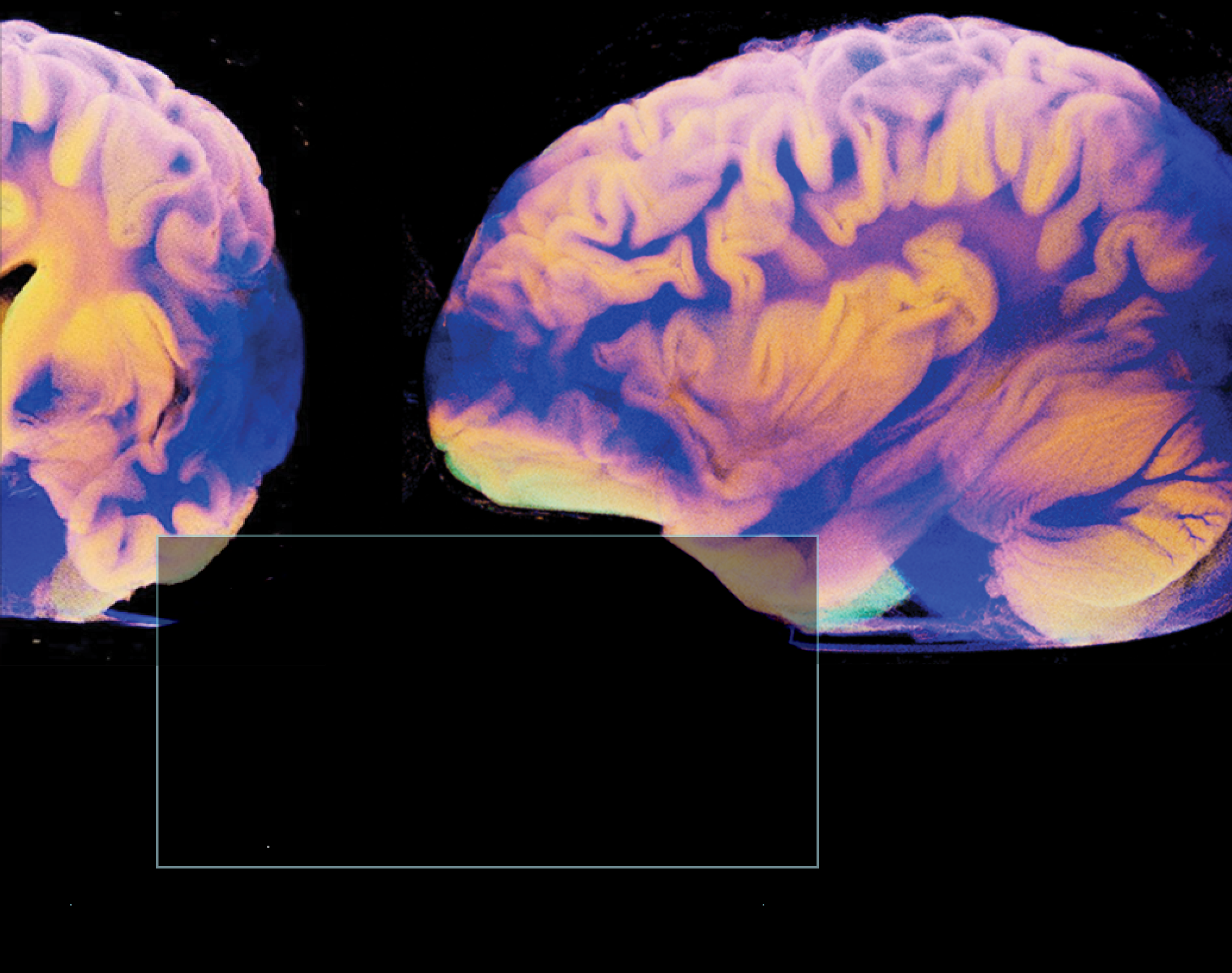
The brain changes with increasing age, from molecules to morphology.
We found that younger “brain age,” a neuroimaging-based assessment of brain health, is associated with better post-stroke outcomes. Our study shows how the health of the overall brain can protect individuals from the functional consequences of stroke. The healthier the brain is, first, the less likely someone is to have a stroke, and second, the less likely someone is to have poor outcomes if they do have a stroke. These findings could lead to better ways to predict post-stroke outcomes and offer insight into new potential treatment targets to improve recovery.
66


It is estimated that up to 70% of people living with epilepsy could live seizure-free if properly diagnosed and treated.
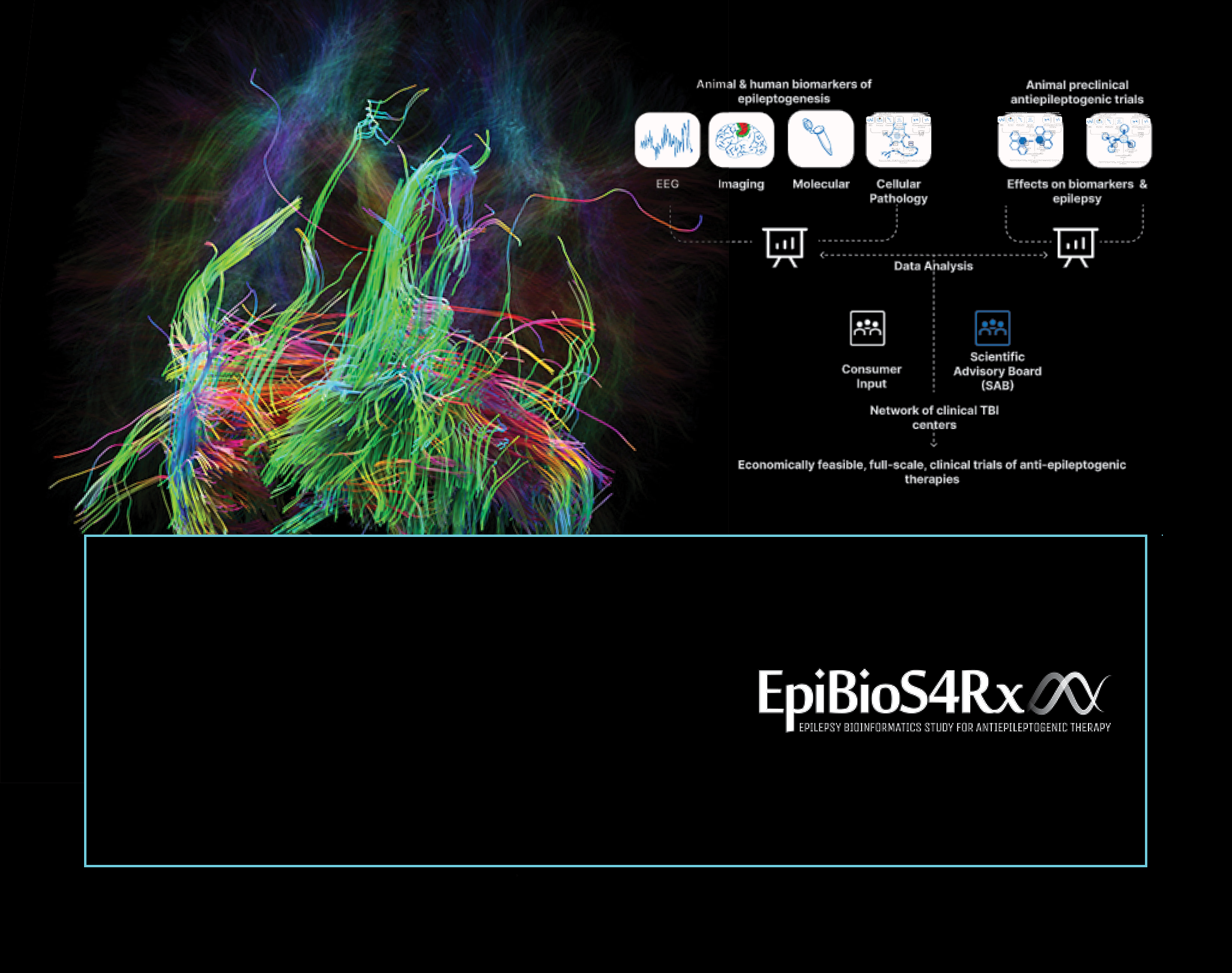
The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) is our extensive, multi-center collaborative effort with the mission of identifying biomarkers that predict subsequent seizures in patients with traumatic brain injury. We identify biomarkers and epileptogenesis in animals and patients, standardize preclinical trials of potential antiepileptogenic therapies, and create open and shared resources for the entire epilepsy community.
67

Older brain age, possibly due to post-stroke atrophy, may reflect a limited capacity for brain repair and recovery.
We created a new way to measure how the brain ages in stroke patients. We looked at brain scans and data from hundreds of stroke patients and used a computer model to estimate the age of different parts of the brain. This model was trained on data from thousands of people without stroke. We found that the size and location of a stroke affected how the brain aged, especially in certain networks related to movement and attention. Damage to a network called the salience network had the biggest impact on aging in other networks. Our findings suggest that including information from brain scans could help predict how well stroke patients recover their movement abilities.
68

Stroke is a leading cause of serious long-term disability. Rehabilitation for stroke patients with severe motor impairments is challenging, but new research indicates that therapies involving virtual reality (VR) and brain-computer interfaces (BCIs) may offer benefits. One of our studies combines VR and BCI in a platform called REINVENT and assesses its effects on chronic stroke patients across different levels of motor impairment. REINVENT acquires post-stroke EEG signals and synchronous electromyography (EMG) data to monitor overt muscle activity. Initial results suggest the safety and potential efficacy of EEG-based BCIs, particularly for severe impairments, while EMG-based feedback may be more suitable for milder impairments.
69

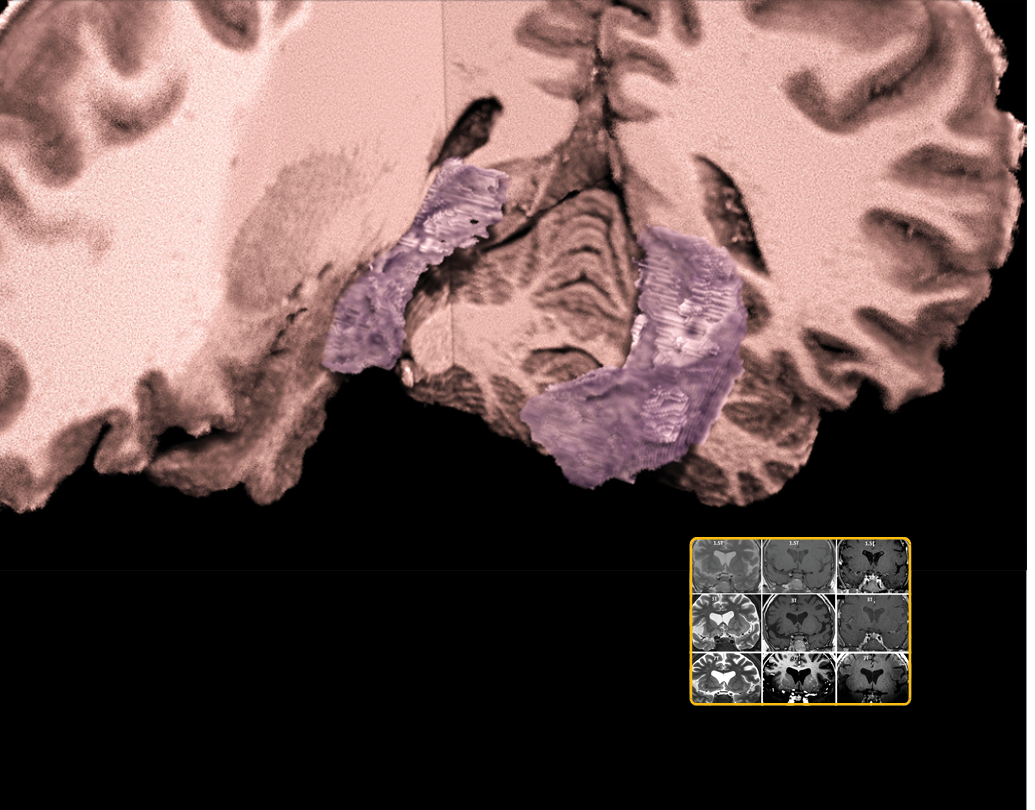
Accurate lesion segmentation is a critical component of stroke rehabilitation research.
We compiled, archived, and shared one of the most extensive open-source datasets of MRI scans from stroke patients. The Anatomical Tracings of Lesions After Stroke (ATLAS) is being used to train algorithms that automatically process MRI images from stroke patients. This technology may be able to forecast which patients will respond to various rehabilitation therapies and personalize treatment plans accordingly.
70


7T MRI can improve lesion detection in about 25% of patients presenting with negative MRI at lower fields.
We have partnered with the Keck Hospital of USC by extending the use of our 7T MRI scanner for the clinical scanning of patients with epilepsy. Our 7T MRI scanner can better detect epileptogenic lesions, such as focal cortical dysplasia, compared with conventional 3T MRI. Numerous vexing brain diseases and conditions might be diagnosed more precisely by performing advanced medical imaging using the 7T MRI scanner.
71






One future goal is to implement an artificial intelligence assistant tool in intensive care units that processes real-time clinical data, including blood tests, CT/MRI scans, and EEG. This AI would analyze the data in the context of epileptogenesis, providing detailed reports on potential associations with the development of epilepsy. A team of clinicians would then review these reports to tailor therapy adjustments for each patient, combining personalized and translational approaches. For traumatic brain injuries, we plan to create AI algorithms to analyze neuroimaging data to assist in the detection and characterization of TBI-related abnormalities. These algorithms can help radiologists identify subtle signs of injury, track disease progression, and predict outcomes more accurately.
We also plan to usher in a new era of precision rehabilitation that uses data-driven decision-making based on brain imaging and other noninvasive measures to identify each individual’s most effective rehabilitation strategies. A personalized regimen of therapeutic activities, including noninvasive brain stimulation, robotic therapy, exercise, brain-computer interface training, and more, could be prescribed based on each person’s unique needs and goals to help them achieve their maximum recovery potential and return to a fulfilling life.
72


73




With a magnetic field strength of 7 Tesla, more than four times that of MRI scanners used in most hospitals, the ultra-high-field 7T Terra magnetic resonance imaging (MRI) machine has a strong signal-to-noise ratio, allowing researchers and clinicians to collect images of the human brain with higher spatial resolution and better contrast than was previously possible. In a typical 1.5 Tesla scanner, each cubic unit of the image — or ‘voxel’ — contains about 100,000 brain cells. At 7 Tesla, a voxel depicts just a few thousand cells, allowing scientists to study the brain with more precision and detail.
7T MRI makes many new features visible to researchers that would otherwise not be visible at a standard field strength of 1.5 and 3T. To date, clinical benefits of 7T MRI have been shown in a range of neurologic disorders, including epilepsy, multiple sclerosis (MS), planning for deep brain stimulation (DBS), cerebral small vessel disease (cSVD), as well as musculoskeletal (MSK) imaging.
Life-saving imaging
Methods to treat cancers
Hidden brain functions revealed









We push the boundaries of what is possible by using ultra-high-field imaging to investigate brain structure, function, and connectivity of the human brain across the lifespan in health and disease. Once images are collected, our researchers use a range of sophisticated tools to gain insights from the data and make those findings accessible to others.The powerful tools and techniques we develop enable breakthroughs in both the lab and the clinic.
STAMP OF APPROVAL
On October 24, 2018, our ultra-high-field 7T Terra magnetic resonance imaging (MRI) scanner received FDA approval for clinical use, opening new avenues of care for patients with Alzheimer’s disease, multiple sclerosis, and other diseases that affect the brain. The scanner, the first of its kind in North America, was installed in February 2017. On October 21, 2019, our scanner became the first 7T in the country to receive accreditation from the American College of Radiology (ACR). ACR Accreditation helps assure our patients that we provide the highest level of image quality and safety by documenting that our facility meets requirements for equipment, medical personnel, and quality assurance.
76


SEEING IT THROUGH
We performed the first ultra-high-field scan of a Cushing’s disease patient in the US. Using our 7T MRI scanner, we were able to localize a pituitary tumor not visible on 1.5T or 3T scanners, which was eventually removed by a team at USC. Cushing’s disease can occur when a benign tumor in the pituitary gland, a pea-sized organ in the brain, causes the gland to secrete too much adrenocorticotropic hormone. This, in turn, triggers the adrenal glands to overproduce cortisol, which can lead to many unpleasant symptoms.
77

ACROSS THE DIVIDE
We developed a new method called zoomed 7T perfusion functional MRI (fMRI) that can help with the complex and intricate task of mapping brain activity, which is key to understanding dynamic activities of neural circuits — connections between brain cells across cortical layers and among brain regions. Compared to previous MRI methods at lower field, the new technique can measure blood flow on a fine-grained scale, enabling researchers to remove unwanted signals such as those from surface-level arteries and veins. Obtaining an accurate read-out of activity from region to region across cortical layers can help researchers understand human brain function in greater detail.
78































































.svg)





BOOSTING IMMUNITY
We created a new cell-labeling technique to improve the visualization capacity of MRI. While each voxel in an MRI image typically represents tens of thousands of cells, we were able to view clusters of less than 100 cells. We labeled macrophages, a type of white blood cell involved in the immune response, with three FDA-approved compounds—heparin, protamine, and ferumoxytol. We then used the 7T to capture images of the macrophages. In some trials, we detected as few as 62 cells labeled with the three compounds. This method can be used to monitor the effectiveness of immune and stem cell therapies used to treat certain cancers.
79


DISCOVERY IS IN THE DETAILS
We have installed an innovative modular body coil (UniQuest, Queensland, Australia) consisting of eight individual antenna elements that allow it to be conformed across multiple body regions. Initial testing on our 7T demonstrated detailed images of nerves or neurography in the pelvis. This body coil opens up numerous opportunities, including highly targeted images and more efficient and faster imaging methods, as the new coil allows targeted examination of parts of organs and more with increased detail.
80


FORMING AN ALLIANCE
We initiated the 7T Translational Alliance of North America (7TANA), which currently consists of over 30 sites in the US and Canada. The mission of 7TANA is to translate the powerful imaging capabilities of 7Tesla MRI to real-life benefits for patients, healthcare providers, and researchers worldwide. The 7TANA provides resources for patients and caregivers seeking 7T MRI, facilitates the standardization of imaging protocols, sharing of data and expertise, and formation of multi-site trials for the discovery and evaluation of clinical values using 7T MRI.
81




LIFESAVERS
With the help of our 7T MRI scanner and a team of experts at USC, a pregnant woman with a glioma brain tumor was able to deliver a healthy baby, and she is currently living with no evidence of disease. This collective effort brought together our imaging specialists with neurosurgery, oncology, and obstetrics and gynecology to save two lives and to showcase the power of translational medicine.
82


We will bridge the existing gap between understanding the microscopic interactions of individual cells and complex neural circuits and the ability to capture and visualize these dynamics in real time and space within living organisms. Our research focuses on investigating the in vivo pathophysiology and identifying early imaging biomarkers for several socioeconomically significant neurological disorders, including Alzheimer’s disease, stroke, small vessel disease, traumatic brain injury, brain tumors, epilepsy, and multiple sclerosis. Our motivation is to translate state-of-the-art research findings into clinical neuroimaging, particularly at 7T. Looking ahead, our methods, exemplified by 7T laminar multi-contrast fMRI, show great potential for academic exploration and clinical applications. This approach is poised to transform our comprehension of the brain, providing insights into neural processes and disorders that were previously elusive. Our ongoing efforts are dedicated to refining this technique and others, aiming to furnish essential tools for future breakthroughs in neuroscience and fostering innovative medical diagnostics and therapies.
83






Premier master’s degree in brain imaging and informatics
Esteemed and long-running postdoctoral fellowship program
Educational outreach for various communities
Our one-year M.S. program in Neuroimaging and Informatics prepares scientists for rewarding careers in biomedical research. The overall goal of our program is to prepare students for a career that involves analyzing neuroimaging data, such as research positions in industry or academia with or without additional schooling. We also provide workshops, seminars, and online educational tools to train researchers in the latest imaging and informatics technology. Participants in these programs receive continuing education, training, and research experience to prepare them to be changemakers in the field.














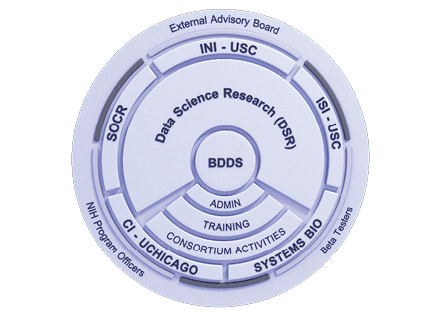

Big Data for Discovery Science Center (BDDS) is our NIH-supported collaborative project that trains investigators worldwide on how to maximize existing datasets while providing them with crucial resources. BDDS provides seminars and an intuitive training system of lectures, tutorials, and more to train the next generation of scientists.
As a part of USC’s Center for Knowledge-Powered Interdisciplinary Data Science program, we led a group of master’s students in learning hands-on applications of artificial intelligence in medical imaging. The students develop critical skills for working with biomedical images, including managing a large, complex dataset of thousands of brain scans.
86

We partnered with California State University Fullerton (CSUF) on the Big Data Discovery and Diversity-Research Education Advancement and Partnership (BD3-REAP) program, which provides enriching research experiences and opportunities through an exploration and understanding of data science to improve health. BD3-REAP provides opportunities for students to gain skills in managing and conveying biomedical information to various communities.
Our current Diversity in Aging NIH R25 training grant, Neurocognitive Aging & Analytics Research Education (NAARE), is a training endeavor in collaboration with California State University Fullerton (CSUF). We develop curricula to teach various aspects of aging, Alzheimer’s disease and related dementias, and research methods to a selected cohort of undergraduate students from backgrounds typically underrepresented in science.
87

In 2020, we added two new classes to our NIIN program. These included our Data Science in Neuroimaging elective, designed to teach students basic Python programming and data science techniques for performing complex analyses using neuroimaging data. We also added a one-credit Science Communications class, which focused on teaching students career skills and to communicate effectively both orally and in writing. We created a coding “boot camp,” which has since grown to include computer setup and coding in Bash, Python, and Matlab.
We proudly host USC’s Master of Science in Neuroimaging and Informatics (NIIN) program. The NIIN program has graduated 121 students since its launch in 2015. In this one-year USC Master of Science degree program, students attend lectures and perform hands-on activities that teach them to acquire brain MRI data on our Siemens 3T and 7T scanners, analyze multimodal brain imaging data, design neuroimaging studies, perform computational modeling, use animal model techniques, and more.


88














































































































































































































































































































































We host a virtual Neuroimaging Seminar Series for our master’s students and the neuroimaging community. The talks, given by a diverse group of expert speakers, are focused mainly on neuroimaging and data science methods or on applications of these methods to clinical and psychological questions. We have maintained a NIH Institutional Training Grant (T32) for over six years.
This two-year postdoctoral fellowship opportunity provides continuing education, experiential training, and hands-on research to prepare for a successful independent research career. Training includes lab rotations, regular seminars, and instruction for teaching and grant writing. The focus is on the analysis of Alzheimer’s disease biomarkers using multiple scales and methodologies, with an emphasis on informatics and translational research.

89


Every year, we host exemplary high school students from the Bovard Scholars program. USC established Bovard Scholars to empower outstanding high school students with financial needs to dream big. They receive resources to help prepare them for success in college, including gaining admission and scholarships to the best schools that are the ideal fit for their personal and professional needs.
90


Through two National Science Foundation grants, Research Experiences for Teachers (RET) and Research Experiences for Undergraduates (REU), we work with high school teachers and undergraduate students to provide crucial hands-on research experience with an emphasis on aggregating multimodal and longitudinal data, curating and harmonizing data, performing data analysis, and teaching machine learning and other analysis methods.
91

We plan to build relationships with non-academic companies and organizations in the area to help students gain internships that will allow them to experience working in industry or with a foundation. We also continue to build our alumni network to help current and previous students collaborate.
With the advancement of ultra-high-field MRI (UHF-MRI), we can view the brain with unprecedented levels of detail, leading to significant neuroscience breakthroughs. With our Institute being a founding member of the 7Tesla Translational Alliance in North America (7TANA), we plan to establish training courses and inter-site exchange programs to train the next generation of UHF-MRI specialists.
92


93




Creating innovative visual tools and technologies
Making scientific findings accessible to all communities
Fostering a connection between basic science and creative arts
We place a unique emphasis on the importance of visual communication to a variety of audiences. We have a dedicated Scientific Visualization Group (SVG) of graphic design, medical illustration, communication, and technology experts who create award-winning 3D models, develop explanatory animations based on real data, and provide stunning and accurate images of the brain to researchers and the greater community.





VISUALIZING BIG DATA
We house significant amounts of data and information at the Stevens INI, and visualization is a tool that transforms massive amounts of numbers into something we, as humans, can quickly and intuitively understand. We use this knowledge to guide the computational approaches we apply to the data, our algorithms, our deep-learning approaches, and more. For example, even a simple MRI scan often consists of nearly 17 million numbers—an MRI scan is often a 3D cube 256 voxels high, 256 voxels wide, and 256 voxels long. We understand these 17 million numbers by looking at them as graphical representations, where the smaller numbers we visualize as darker and the larger values we visualize as brighter. That is what a ‘typical’ MRI image is.
96


SPEAKING VOLUMES
We create unique volumetric dataset visualizations for presentations and communication. The data are rendered using 3D animation software and other tools that are not typically used for medical imaging, providing us with innovative ways to view and examine the data, which we then share with the scientific community and beyond.
97



REALITY CHECK
Schol-AR is a powerful web and smartphone application we created that uses augmented reality (AR) to revolutionize how research and educational outputs are shared. Scientists are using the app to embed 3D models, videos, and other data in their posters, publications, and presentation materials to improve engagement and the explanatory power of their work.
Augmented Reality
PDF Reader
Schol-AR augmented articles have been published in:




Paper,
Poster, or
Other Format
Upload Content
Browser & Mobile Compatible
Include Provided QR Code

Image Stacks
3D Models
Volumes
More Coming
Video
98



COMMITMENT TO THE PUBLIC
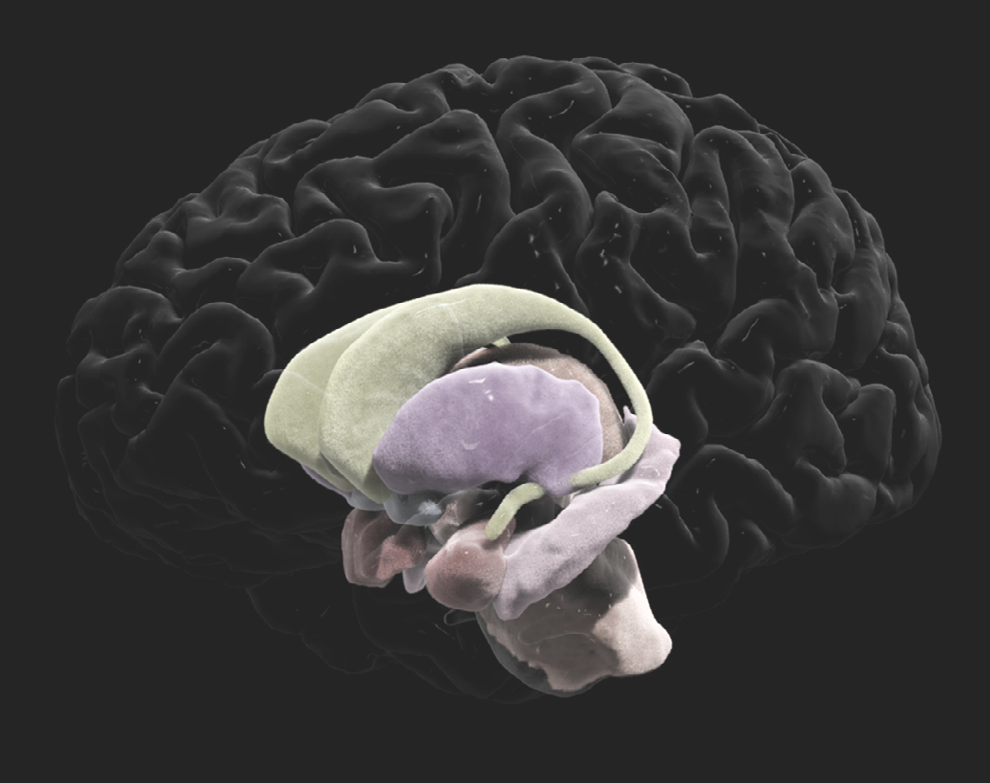
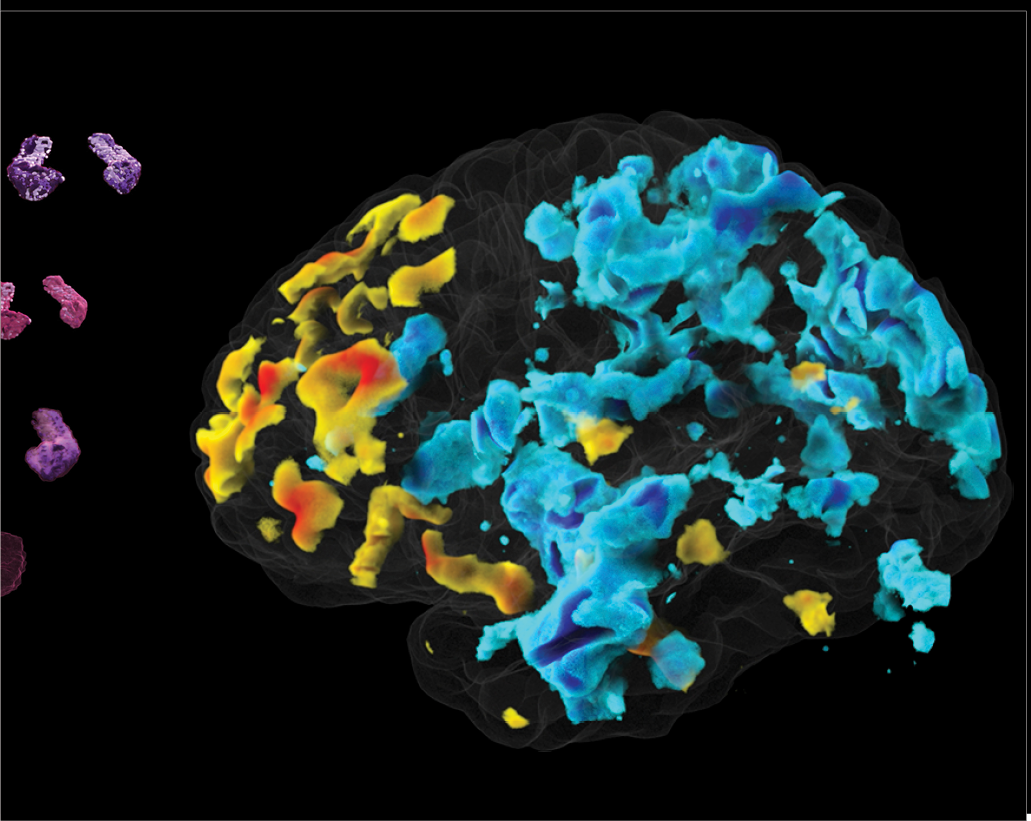
We constantly use our visualization work for public outreach by creating visualizations of our data with informative voiceovers for lay audiences. Our goal is to inform the general public about how research is progressing with tools that make very complex research more approachable and understandable. The data shown in this image were collected using genetic engineering
techniques and a powerful two-photon microscope. It depicts neuronal connections between the amygdala, which is responsible for emotions and feelings of fear, and the medial prefrontal cortex. By isolating connections to and from specific areas of the brain, scientists can more closely investigate how these regions form the neural circuits that drive our behavior.
99
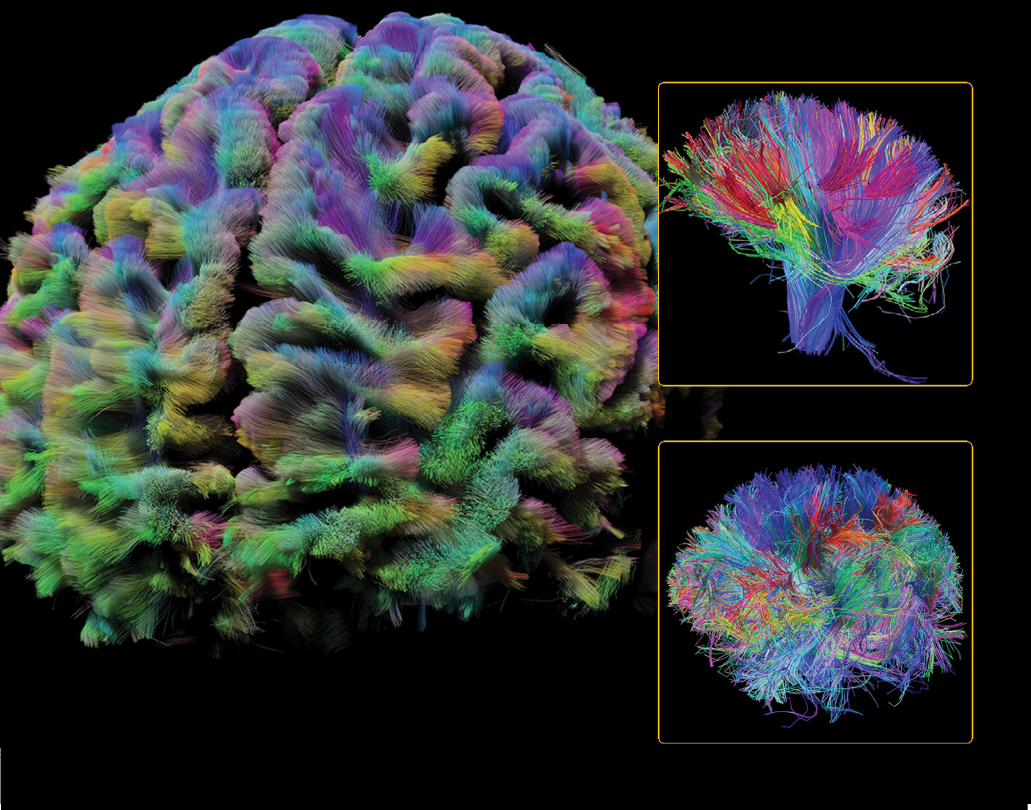
ALL OVER THE MAP
We were one of the first two awards of the NIH’s Human Connectome Project (HCP), an ambitious effort to map the neural pathways that underlie human brain function. The project’s overarching purpose is to acquire and share data about the structural and functional connectivity of the human brain. We pioneered visualization techniques that create stunning maps of MRI diffusion spectrum imaging-based tractography of fibers crisscrossing the brain.
100


DOWN TO A FINE ART
Traditional medical illustration has an essential role in teaching the next generation of scientists and facilitating collaboration between disciplines. The brain is incredibly complicated, and medical illustration is often used to isolate certain parts of the brain for closer inspection. We continuously improve existing 2D illustrations and techniques to contribute to these vital educational tools.
101
DOING THE MATH
We also use visualizations to better illustrate and demonstrate the physics, mathematics, and computational techniques we use to understand and investigate data. For example, we can graphically show computational techniques such as deconvolution to help better explain and understand how they are used on MRI data. These complex mathematical techniques are fundamental for many of our MRI methods. Deconvolution—a process that can be used to resolve something into its constituent elements in order to clarify it—is used in determining fiber orientations for tractography, which, in turn, is how we discover how brain regions and circuits are connected.
102


ARTISTIC LICENSE
We are proud to create neuro-art, the intersection of neuroscience and artistic expression. We believe this holds significant importance in unraveling the mysteries of the human mind and fostering a deeper connection between science and creativity. This symbiotic relationship between art and neuroscience not only enhances our understanding of the intricate workings of the brain but also provides novel insights into the subjective nature of artistic perception. Neuro-art has the potential to bridge gaps between disciplines, fostering collaboration between artists and neuroscientists to provoke thought, encourage discussion, and expand worldviews.
103

The field of data visualization is poised for transformative growth and innovation. Advancements in technology, particularly in augmented and virtual reality, will revolutionize how we interact with and interpret data. Immersive and interactive data experiences will become more commonplace, allowing users to explore complex datasets in 3D spaces. Machine learning and artificial intelligence will play a pivotal role in automating the process of turning raw data into insightful visual representations, enabling faster and more accurate decision-making. The integration of real-time data will enhance the dynamic nature of visualizations, providing up-to-the-minute insights. The next decade holds exciting prospects for data visualization, promising a convergence of cutting-edge technologies and thoughtful design to unlock new dimensions of information understanding.
104


105

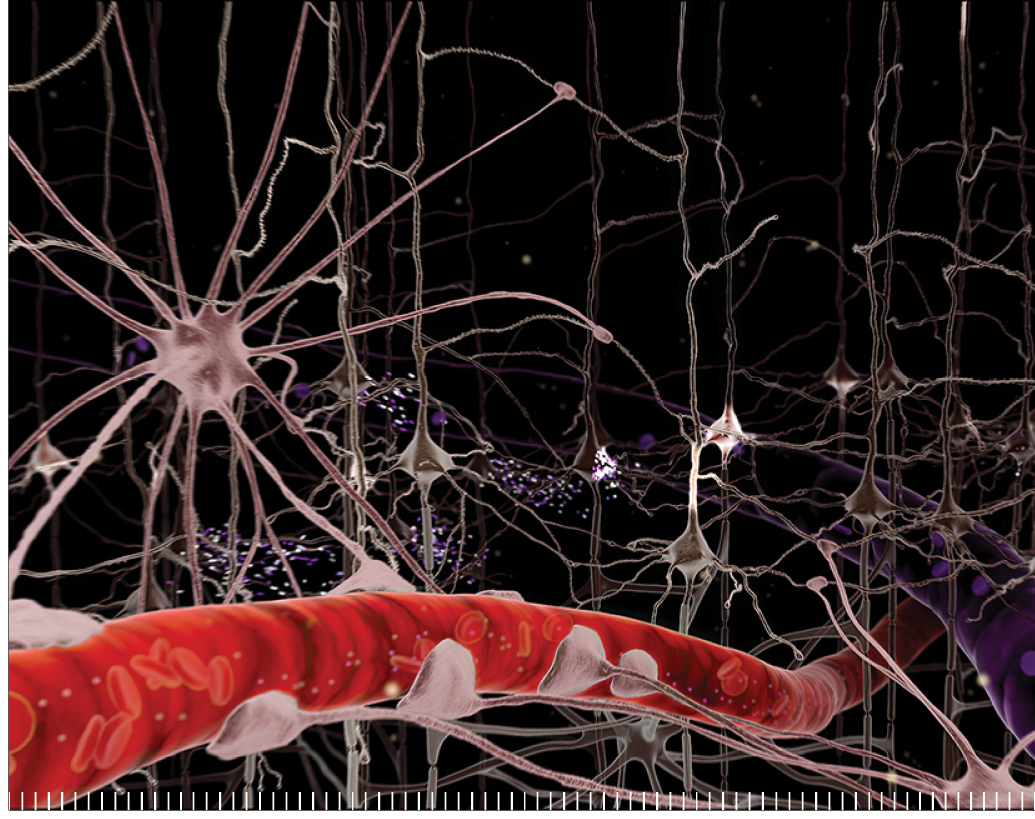




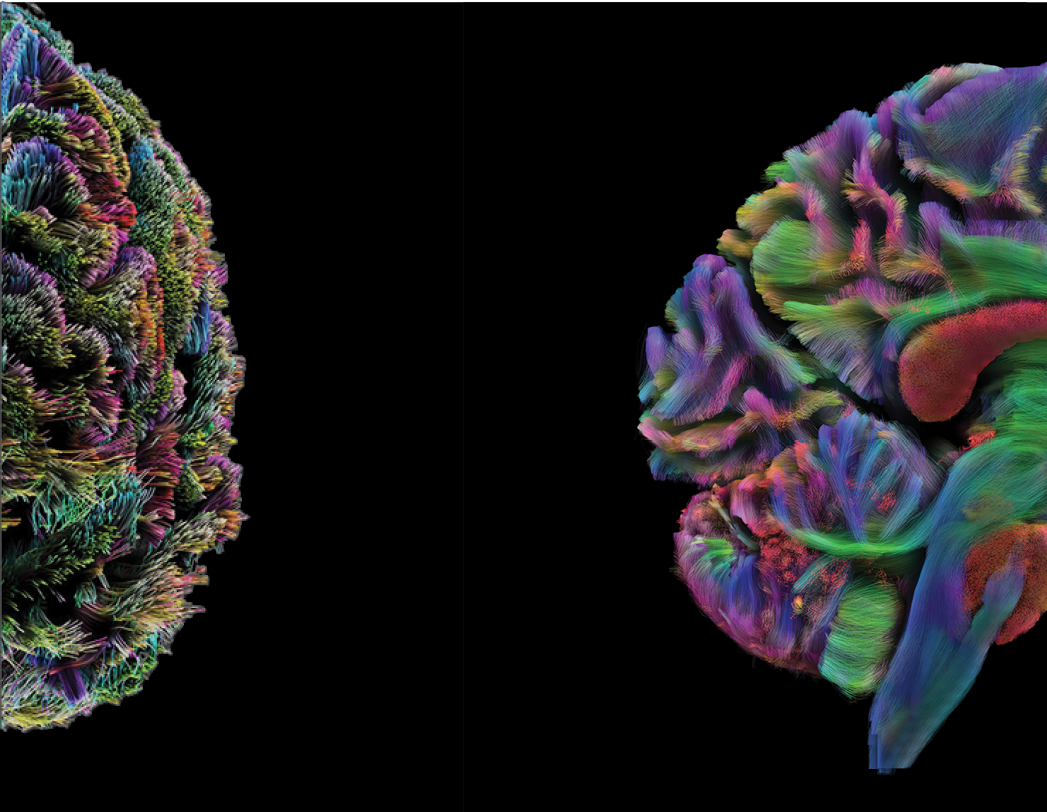

High-resolution imaging will enable the visualization of intricate neural pathways,
providing unprecedented insights into the brain’s structural connectivity. Integration with functional imaging and the application of machine learning algorithms will yield a more holistic understanding of the complex interplay between brain structure and function. Standardization and validation efforts will enhance the reliability of tractography results, facilitating its integration into clinical settings for personalized diagnostics and treatment strategies.
108


Precision gene-editing techniques will enable the creation of increasingly sophisticated mouse models,
allowing scientists to replicate specific genetic mutations associated with neurological disorders. Advanced imaging technologies will offer unprecedented insights into the mouse brain’s activity, offering a more nuanced understanding of neural circuits and behaviors. Collaborative efforts and open-access data initiatives will accelerate discoveries, resulting in a comprehensive, multimodal map of the mouse brain in all its complexity. As these models become more refined and translatable to human conditions, insights into the molecular and cellular underpinnings of neurological diseases will guide the development of targeted therapeutic interventions.
109
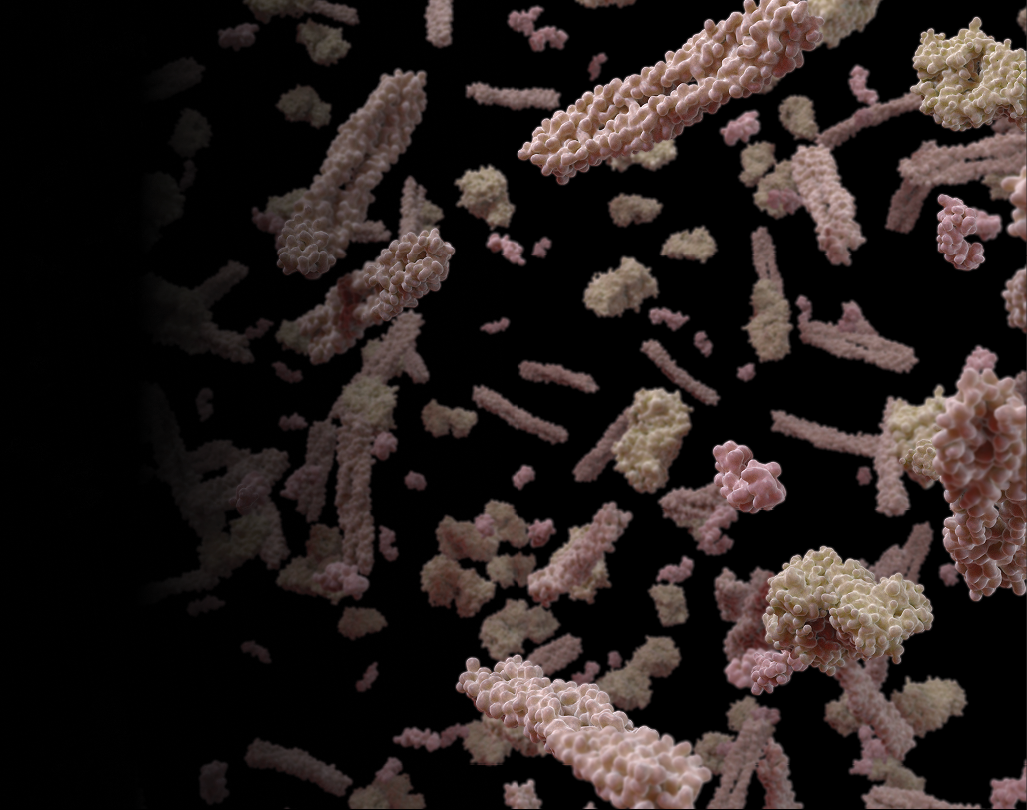
The discovery of plasma-based biomarkers for neurodegenerative disorders will enable numerous revolutionary medical breakthroughs.
These biomarkers will provide scalable, practical, non-invasive, and cost-effective early detection of conditions like Alzheimer’s or Parkinson’s, facilitating timely intervention and personalized treatment strategies. Routine blood tests could screen individuals for potential risk, enabling a proactive approach to managing these debilitating disorders. Simple blood test-derived biomarkers will help enhance our understanding of disease progression, paving the way for targeted drug development and more effective and timely therapeutic interventions.
110


High-resolution imaging techniques paired with real-time monitoring
of blood flow, oxygen levels, and microvascular changes will offer a comprehensive view of neurovascular coupling and its role in brain function. These breakthroughs in vascular imaging will deepen our understanding of normal brain physiology and shed light on the vascular contributions to neurological disorders, paving the way for innovative diagnostic tools and targeted therapies. Integrating vascular imaging with other modalities will likely lead to a more holistic approach to studying brain health and may uncover novel avenues for treating and preventing neurological conditions.
111
Machine learning algorithms will play a pivotal role in extracting meaningful patterns
from the vast sea of data, uncovering intricate relationships between genes, neural circuits, and behaviors. Massive datasets generated from neuroimaging, genomics, and other methods will be seamlessly integrated through sophisticated informatics platforms. Collaborative, open-access initiatives will facilitate data sharing, fostering a global knowledge network. This integration of big data and informatics will accelerate our understanding of the brain’s complexity and enable personalized approaches to neurological disorders, tailoring treatments based on an individual’s unique biological profile.
112


For stroke and seizure disorders, personalized treatment plans will be crafted
based on a comprehensive understanding of an individual’s genetic makeup, neuroimaging data, and specific risk factors. Breakthroughs in neurostimulation techniques and targeted drug delivery systems will provide more effective and tailored interventions for seizure control and stroke recovery. Real-time monitoring devices and wearable technologies will enable continuous patient tracking, facilitating early detection and intervention. Collaborative research and data-sharing initiatives will contribute to a deeper understanding of the underlying mechanisms, paving the way for novel therapeutic targets and offering more precise, timely, and patient-centric solutions.
113

With increasingly powerful magnetic fields in ultra-high-field MRI, imaging resolution will reach new heights,
enabling the detection of subtle anatomical and functional variations,
which will be invaluable in characterizing unique biological signatures and tailoring treatment strategies to each patient’s specific needs. Ultra-high-field imaging will be pivotal in identifying early disease markers, monitoring treatment responses, and predicting individual patient outcomes. Integrating this advanced imaging technology with genomic, proteomic, and clinical data will create a comprehensive understanding of each patient’s health profile, ushering in a new era where medical interventions are precisely tailored to maximize efficacy and minimize side effects, ultimately transforming the landscape of personalized medicine.
114


Virtual and augmented reality platforms will offer immersive experiences in neuroscience education,
allowing students to explore and interact with 3D brain structures and neural circuits previously impossible ways. Advanced simulations and virtual laboratories will provide hands-on training, enhancing practical skills in research and diagnostics. Collaborative online platforms and open-access resources will facilitate global knowledge sharing and networking among neuroscience professionals and students. This interdisciplinary and technology-driven transformation can also democratize access to neuroscience education, fostering a new generation of skilled and diverse neuroscientists with the tools to tackle complex brain research and healthcare challenges.
115
116

As all aspects of neuroimaging advance, so must our scientific visualizations to develop explanatory images and animations for researchers and the general public. This will require improving our visualizations to include more and larger data as our datasets combine and expand, as well as improvements in image resolution and carefully designed visualization strategies that focus on intuitively displaying critical information. The future of scientific visualization will also be further transformed by artificial intelligence. AI algorithms will enable dynamic and context-aware visualization, allowing researchers to intuitively navigate and interpret complex datasets. Additionally, AI-driven tools will facilitate real-time data analysis, enhancing the interactive exploration of intricate scientific phenomena. These developments will accelerate the extraction of meaningful insights from diverse datasets and empower researchers across disciplines to communicate and comprehend complex scientific concepts more effectively, fostering a new era of discovery and collaboration.
117




USC Mark and Mary Stevens
Neuroimaging and Informatics Institute
Keck school of USC
2025 Zonal Avenue, Los Angeles,CA 90033
Phone: (323)44-BRAIN(442-7246)
info@ini.usc.edu
A Decade
USC establishes INI with a founding team of 120 faculty, staff and student from LONI
Mark and Mary Stevens make a transformational gift to the INI that supports the custom design and buildout of Stevens Hall for Neuroimaging.
One of the world’s most powerful MRI scanners, a Siemens Terra 7T, is installed. The 7T is the first ultra-high field scanner of its kind installed in North America.
2
0
1
3
2
0
1
5
2
0
1
7
2
0
2
3
DISCOVERY
of